Endless Improvements
العربية
Mineral Base Oil Composition
Crude oil results from physical and chemical processes acting over many million years on the buried remains of plants and animals. Although crude oil is usually formed in fine-grained source rocks, it can migrate into more permeable reservoir rocks and large accumulations of petroleum, the oilfields, are accessed by drilling.
Each oilfield produces a different crude oil which varies in chemical composition and physical properties.
Some crude oils, have a low sulphur content and flow easily.
Others may contain wax and flow only when heated.
Others contain very large amounts of very high molecular weight asphalt
Despite the wide range of hydrocarbons and other organic molecules found in crude oils, the main differences between crudes are not the types of molecules but rather the relative amounts of each type that occur in each crude oil source.
Components of Crude Oil:
The components of crude oil can be classified into a few broad categories. Some of these components have properties desirable in a lubricant whilst others have properties which are detrimental.
1. Hydrocarbons:
Organic compounds composed exclusively of carbon and hydrogen predominate in all crude oils. They can be further subdivided into the following:
a. alkanes: known as paraffins, with saturated linear or branched-chain structures.
Structure:
[Linear Alkane]

[Branched Alkane]

b. alkenes: known as olefins, unsaturated molecules, but comparatively rare in crude oils. Certain refining processes produce large amounts of alkenes by cracking or dehydrogenation.
Structure:

c. Alicyclic: known as naphthenes, are saturated cyclic structures based on five- and six-membered rings.
Structure:

d. Aromatics: cyclic structures with conjugated double bonds, mainly based on the six-membered benzene ring.
Structure:
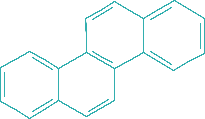
This is a simplified classification because many hydrocarbons can be combinations of these classes, e.g. alkyl-substituted cyclic or mixed poly-cyclics containing both aromatic and fully saturated rings.
2. Heterocyclic compound:
Many organic compounds in crude oil incorporate other elements, sometimes within ring structures or as functional groups attached to a hydrocarbon structure.
a. Organo-metallic Compounds: Within the boiling range appropriate to lubricant base oils, almost all organo-sulphur and organo-nitrogen compounds are heterocyclic molecules
Organo-sulphur compound:
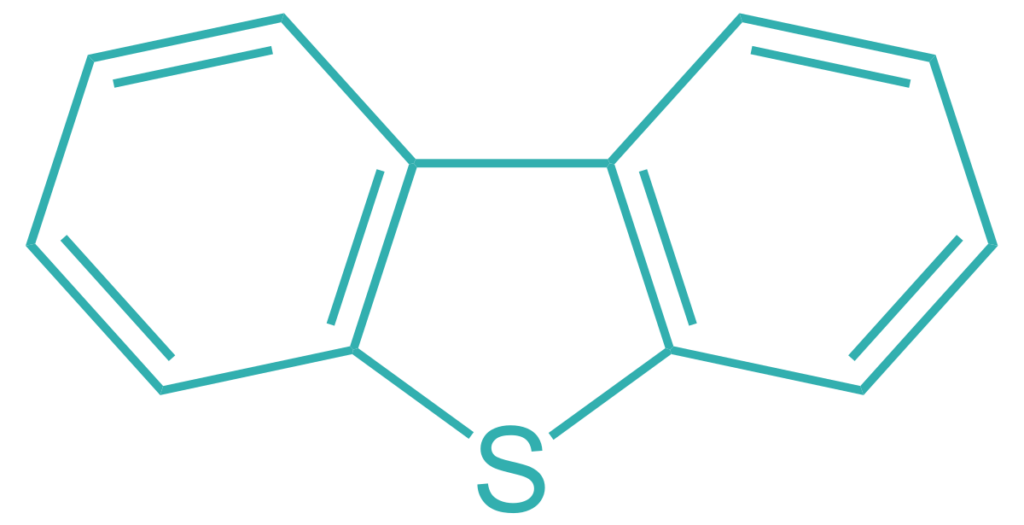
Organo-nitrogen compound:

b. Oxygen-containing molecules: are carboxylic acids as either saturated aliphatic acids or cycloalkanoic acids (naphthenic acids).
Traces of phenols and furans may also occur.
3. High Molecular Weight Component:
There are very high molecular weight resins and asphaltenes which contain a variety of aromatic and heterocyclic structures.
a. Resins: are the lower molecular weight, <1000 amu, species.
b. Asphaltenes: result from linking together many other structures and have exceptionally high molecular weights.
Characteristics of the Hydrocarbons for Lubricant Performance:
Only hydrocarbon properties are discussed in this section because most of the non-hydrocarbons are prone to oxidation or degradation and are deleterious to lubricant performance. However, organo-sulphur molecules are known to act as naturally occurring antioxidants and it is frequently desirable to retain some of these in a refined base oil.
Alkanes, alicyclic and aromatics of the same molecular weight have markedly different physical and chemical characteristics. Physical characteristics affect the viscometrics of the lubricant, and the chemical stability of each class to oxidation and degradation is very important in use.
Alkanes
Of the three main classes, alkanes have relatively low densities and viscosities for their molecular weights and boiling points. They have good viscosity/temperature characteristics, i.e. they show relatively little change in viscosity with change in temperature compared to cyclic hydrocarbons. However, there are significant differences between isomers as the degree of alkane chain branching increases.
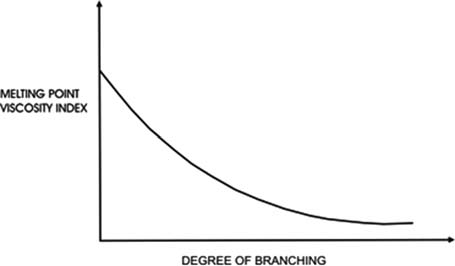
Linear alkanes, the ‘normal’, or n-paraffins in the lubricant boiling range have good viscosity/temperature characteristics but their high melting points cause them to crystallize out of solution as wax.
In contrast, highly branched alkanes are not waxy but have less good viscosity/temperature characteristics.
There is a compromise region in which acceptable viscosity index, VI, and acceptable low temperature properties are achieved simultaneously. In general, alkanes also have good viscosity/pressure characteristics, are reasonably resistant to oxidation and have particularly good response to oxidation inhibitors.
Alicyclics
Alicyclics have rather higher densities and viscosities for their molecular weights compared to alkanes. An advantage of alicyclics over alkanes is that they tend to have low melting points and so do not contribute to wax. However, one disadvantage is that alicyclics have inferior viscosity/temperature characteristics.
Single-ring alicyclics with long alkyl side chains, however, share many properties with branched alkanes and can be highly desirable components for lubricant base oils. Alicyclics tend to have better solvency power for additives than pure alkanes but their stability to oxidative processes is inferior.
Aromatics
Aromatics have densities and viscosities which are yet still higher. Viscosity/temperature characteristics are in general rather poor but melting points are low. Although they have the best solvency power for additives, their stability to oxidation is poor.
As for alicyclics, single-ring aromatics with long side chains, alkylbenzenes, may be very desirable base oil components.
Crude Oil Selection for Base Oil Manufacture:
Different crude oils contain different proportions of these classes of organic components and also vary in the boiling range distribution of their components. The main factors affecting crude oil selection for the manufacture of base oils are the following:
Content of material of a suitable boiling range for lubricants.
Yield of base oil after manufacturing processes.
Base oil product properties, both physical and chemical.
The manufacturing process at a base oil refinery consists of a series of steps to separate the desirable lube components from the bulk of the crude oil. In brief their aims are as follows:
Distillation: removes both the components of too low boiling point and too high boiling point, leaving the lubricant boiling range distillates.
Aromatics removal: leaves an oil that is high in saturated hydrocarbons and improves VI and stability.
De-waxing: removes wax and controls low-temperature properties of the base oil.
Finishing: removes traces of polar components and improves the colour and stability of the base oil.
The yield of base oil after these processes depends on the amount of desirable components in the lubricant boiling range. Lubricant distillates from different crudes can have radically different properties.
| Crude Source | North Sea | Middle East | Nigeria | Venezuela |
| Forties | Arabian | Forcados | Tia Juana | |
| Viscosity at 40°C (cSt) | 16 | 14 | 18 | 23 |
| Pour point (°C) | 25 | 19 | 18 | -48 |
| Viscosity index | 92 | 70 | 42 | 10 |
| Sulphur content (wt%) | 0.3 | 2.6 | 0.3 | 1.6 |
| Aromatic (wt%) | 20 | 18.5 | 28 | 21 |
Both the Forties and Arabian distillates have relatively high VI and high pour point because they are rich in alkanes and are examples of paraffinic crude oils. Paraffinic crudes are preferred for manufacturing base oils where viscosity/temperature characteristics are important, e.g. for automotive lubricants for operation over a wide temperature range. However, there is a big difference in sulphur content between these two crude oils and this has an effect on base oil composition and its chemical properties, especially natural oxidation stability. Careful control of the manufacturing processes can minimize some of these differences.
The Nigerian and Venezuelan distillates are examples of naphthenic products because they are relatively low in alkane content. In particular, the Venezuelan distillate is wax-free and a de-waxing step is not required. Although naphthenic products have inferior viscosity/temperature characteristics, they have other beneficial properties which are particularly useful in industrial applications.
The examples given are all crude oils regularly used to make base oils but many other crudes do not contain sufficient useful lubricant components and cannot be economically used for conventional base oil production.
