Endless Improvements
العربية
Viscosity Index Improvers
Viscosity Index Improvers, also known as viscosity modifiers, are high molecular weight, oil-soluble polymers, which ideally provide increased viscosity at higher temperatures and minimal viscosity contribution at lower temperatures. The ‘viscosity index’, VI, was an important measure of quality early in the history of the lubricants industry, indicating an oil’s potential applications over a wide range of temperatures, Pennsylvania grade oils, ~100 VI, were the standard against which all others were measured. Hydrogenation and solvent extraction processes were developed to upgrade lubricants from poorer quality crude oils, but the practical VI ceiling for 1930s refinery technology was ~110–115.
Early workers found that small amounts of rubber dissolved into mineral oil substantially raised VI; however, high levels of unsaturation in the polymer led to oxidation and sludge formation. This was overcome by using a synthetic polymer prepared from gasoline light ends, and similar behavior was later described for polymethacrylates and polyisobutylene. As these materials were initially and primarily used to increase the viscosity index, they became known as ‘viscosity index improvers’, or VIIs.
A wide variety of polymers have been explored as viscosity index improvers. Five core technologies currently find important commercial usage:
1. Polymethacrylates [PMAs]:
Typically, a methacrylate VII is a linear polymer constructed from three classes (three distinct lengths) of hydrocarbon side chains:
Short-chain alkyl methacrylates: contains C1-C7. The inclusion of such short-chain materials influences polymer coil size particularly at colder temperatures and thus influences the viscosity index of the polymer in oil solutions.
Intermediate-chain alkyl methacrylates: contains C8–C13. These serve to give the polymer its solubility in hydrocarbon solutions.
Long-chain alkyl methacrylates: contains C14 or more. Interact with wax during its crystallization and thus provide pour point depressing properties.
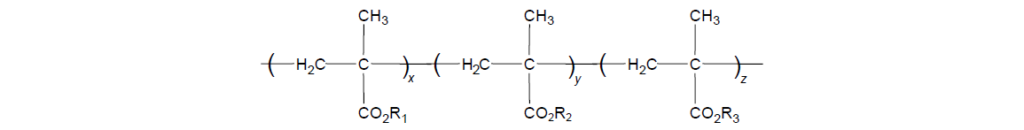
The selected monomers are mixed together in a specific ratio to provide an overall balance of the aforementioned properties. This mixture is then polymerized to provide a copolymer structure in which R represents different alkyl groups and x indicates various degrees of polymerization.
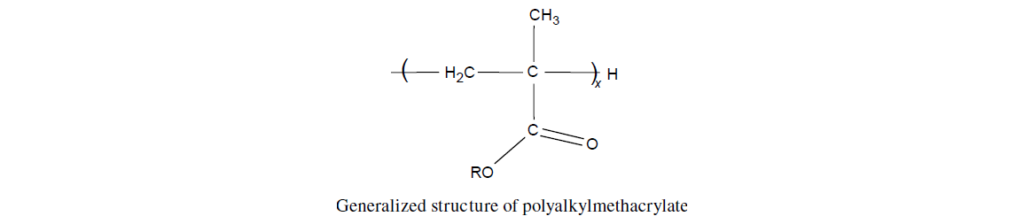
Monomer Chemistry:
Before discussing lubricant additives based on PMA, it is necessary to give an introduction to the chemistry of their parent monomers. The basic structure of a methacrylate monomer is:
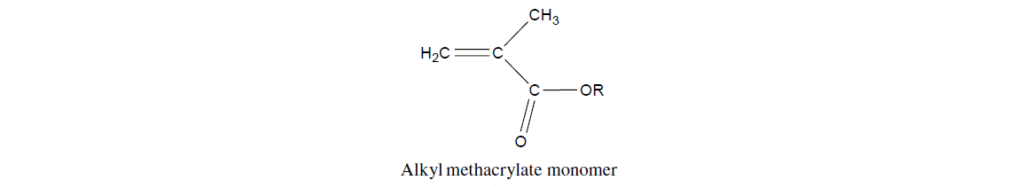
The four salient features of this vinyl compound are as follow:
The carbon–carbon double bond that is the reactive site in addition polymerization reactions.
The ester functionality adjacent to the double bond, which polarizes and thus activates the double bond in polymerization reactions.
The pendent side chain attached to the ester (designated as R). These chains may range from an all-hydrocarbon chain to a more complex structure containing heteroatoms. A significant portion of the beneficial properties of PMAs is derived from the pendant side chain.
The pendant methyl group adjacent to the double bond, which serves to shield the ester group from chemical attack, particularly as it relates to hydrolytic stability.
Polymer Chemistry:
A combination of alkyl methacrylate monomers chosen for a given product is mixed together in specific ratios and then polymerized. Commercial products cover a broad range of polymer molecular weights ranging from ∼20,000 to ∼750,000 Da. Molecular weight is carefully controlled and targeted to produce products that achieve suitable shear stability for a given application.
Higher-molecular-weight PMAs are rather difficult to handle as neat polymers; hence, it is necessary in almost all commercial cases to use a solvent to reduce viscosity to levels consistent with reasonable handling properties. The solvent can be made from higher quality, lower viscosity grade mineral oils. Choice of solvent viscosity primarily depends on the end application; choices range from very low viscosity oils of 35 SUS to light neutrals typically up to 150N. Generally, a higher molecular weight polymer requires more solvent. Commercial products may thus contain polymer concentrations over a very broad range of ∼30 to 80 wt%.
Chemical Properties:
I. Hydrolysis:
PMAs are not very susceptible to hydrolysis reactions; however, the question of hydrolytic stability is often posed because of the presence of the ester group. In the polymeric form, methacrylate ester groups are quite stable since they are well shielded by the surrounding polymer as well as the pendant side chains. The immediate chemical environment surrounding the ester is definitely hydrophobic and not compatible with compounds that participate in or catalyze hydrolytic reactions. There is no evidence that PMA hydrolysis is of any significant consequence in lubricant applications.
II. Thermal Reactions: Unzipping and Ester Pyrolysis
These reactions are known to occur with PMAs, but very vigorous conditions are needed. Thus, there appears to be no important consequences in lubricant applications since bulk oil temperatures are usually lower than the onset temperatures of these reactions. Temperatures on the order of 250°C are needed to initiate the reaction.
II. Oxidative Scissioning
Like any hydrocarbon when exposed to severe oxidative conditions, PMAs can be subject to classic oxidation reactions resulting in polymer scissioning. The scissioning reaction yields two fragments of various lengths each of which is obviously of lower molecular weight than the parent chain, and consequently, there is some loss of viscosity contribution. The reaction takes place at random sites along the backbone since oxidative or free radical attack may occur anywhere along the polymer chain. Allylic, benzylic, or tertiary hydrogen are most susceptible to oxidative or free radical attack. Methacrylates do not normally contain those structural elements; thus, the reaction is not normally an important consideration.
IV. Mechanical Shearing and Free Radical Generation
A well-known, very important degradation reaction of any VII including PMAs is mechanical shearing. Although polymer shearing begins as a physical process, it does generate free radicals. For each polymer chain rupture, two transitory carbon-centered free radicals are generated. In lubricants, the free radicals are apparently quickly quenched, presumably by abstracting hydrogen from the surrounding hydrocarbon solvent or perhaps by the antioxidants in formulated lubricants. Overall, there appear to be few if any further chemical consequences. However, there are important viscometric consequences since the rupture leads to two lower-molecular-weight fragments that provide a reduced viscosity contribution. The shearing process is initiated through the concentration of sufficient energy within the polymer chain to induce homolytic cleavage of a carbon–carbon bond in the backbone of the polymer. The susceptibility of the polymer to mechanical shearing is not related to its chemical structure; rather it is very clearly a function of polymer molecular weight or even more appropriately to the end-to-end distance of the polymer chain. Overall, VII shear stability, although an important physical process, does not appear to carry any appreciable chemical consequences. Further discussion of shearing can be found in the section on the effect of structure on physical properties.
Physical Properties:
VIIs are used to achieve the advantages of multigrade lubricating oils in numerous applications including crankcase engine oils, automatic-transmission fluids, high VI hydraulic fluids, gear oils, and other lubricants used primarily (but not necessarily) outdoors. PMA chemistry dominates applications where high viscosity index and superior low-temperature properties are required.
Polymer molecular size influences thickening at all temperatures, and the larger the coil size, the higher the thickening power. Conversely, with smaller coil size, less thickening occurs. This desirable viscosity–temperature behavior stems from PMA ester functionality imparting polarity to the polymer in the nonpolar hydrocarbon solvent, mineral oil, leading to a relatively small molecular coil size at low temperature. The ester polarity can be accentuated by the use of short pendant side chain monomers.
I. Thickening Properties
Thickening properties for any chemical class of VIIs or viscosity modifier are related to their immensely greater molecular size compared to that of the solvent in which they are dissolved. The long polymeric strand, the backbone of the polymer, is configured in a random coil shape. The overall viscosity of a polymer-thickened solution is related to polymer concentration and molecular weight through the following equation developed by Stambaugh.
ln η = KMva c – k” (Mva)2 c2 + ln η0
| Where; | η: | Overall viscosity | c: | VII concentration | a: | Solubility |
| Mv: | VII average molecular weight | η0: | Solvent viscosity |
For a PMA VII in solution, the coil size expands and contracts with temperature. At lower temperatures, PMA is, on a relative basis, poorly soluble in oil. This is not meant to say that PMA precipitates from solution, but the relatively poor solubility results in a contracted, smaller-volume polymer coil, which has a relatively low viscosity contribution. As temperature increases, solubility improves and polymer coils eventually expand to some maximum size and in doing so donate more and more viscosity. The process of coil expansion is entirely reversible. The polymer coil will continue to expand or contract with temperature changes irrespective of the aging history of the solution . In contrast, nonpolar polymeric thickeners are well solvated by oils at all temperatures and experience far less change to coil size with temperature.
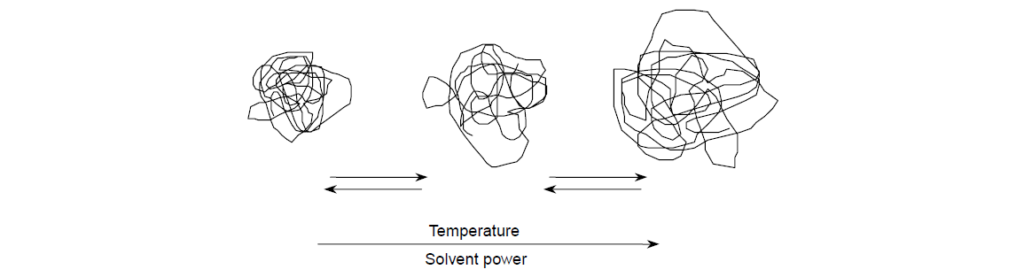
For any VII or VM chemistry, these solubility factors relate to the value of the exponent (a) in the aforementioned equation and, specifically for PMA, are a function of the average length side chain chemistry: short, intermediate, and long alkyl chains.
A typical average side chain length of about eight carbons will provide PMA solubility in oil, even down to extremely low temperatures. Thus, an intermediate-chain length monomer is used to provide overall oil solubility and is normally selected from linear or branched chains composed of 8–14 carbons. The longer chains consisting of more than 14 carbons may be incorporated to provide wax crystallization interactions as described earlier in the section on Pour Point Depressant. Very short chains, usually C1 or C4, are used to balance the composition and make the average chain length at least eight carbons for paraffinic mineral oil solubility. To a very good first approximation, the average side chain length will determine viscosity–temperature properties rather than the detailed nature of the side chain structures.
II. Shearing Effect
Commercial PMA VIIs are available in various chemical compositions and molecular weights ranging from ∼20,000 to 740,000 Da. The higher-molecular-weight materials are not only the most efficient thickeners and provide the greatest VI lift but also the most susceptible to shearing effects. Selection of a suitable VII for a given application should focus on shear stability, thickening efficiency, and VI lift.
Shearing effects, either temporary or permanent, are related to polymer backbone molecular weight. High-molecular-weight polymers are subject to both temporary viscosity loss through shear thinning and permanent viscosity loss when polymer chains are broken in mechanical mastication, both of which result in loss of thickening.
Temporary viscosity loss:
It occurs when polymer molecules become oriented along the axis of flow at sufficiently high shear rates. This phenomenon, known as shear thinning, occurs at a minimum, nominal value on the order of 104 s–1. Shapes of individual polymer coils change from a spherical to an elongated configuration that occupies a smaller hydrodynamic volume and thus contribute less viscosity. With further increases of shear rate, molecules increasingly deform leading to a corresponding greater loss of viscosity contribution until maximum distortion is reached. Within the lubricant community, non-Newtonian, shear-thinning behavior is better known as “temporary loss of viscosity” since the process is reversible upon removal of high shear rates. Distorted polymer molecules resume random coil shapes, reoccupy original hydrodynamic volumes, and contribute viscosity just as before the application of a higher shear rate. The degree of temporary viscosity loss depends on the level of shear stress and the molecular weight (size) of the polymer. Because of their small coil sizes, low-molecular-weight polymers are less susceptible to shear thinning.
The temporary viscosity loss of PMAs is directly related to molecular weight, or even more appropriately, to backbone molecular weight. PMAs are not associative thickeners and do not experience viscosity losses through the loss of molecular associations in high shear stress fields. Any loss of viscosity is related merely to at-rest molecular size and subsequent molecular shape distortion leading to lower hydrodynamic volume.
The data taken from a set of SAE 10W-40 oils blended to constant kinematic viscosity (14.5 mm2/s) and constant cold cranking simulator (CCS) viscosity with the same compounding materials, except for the VII. Three chemically equivalent d-PMA VIIs, differing only in molecular weight, were used in the formulations. The resulting high-temperature, high-shear-rate (HTHS) viscosities are clearly a function of polymer molecular weight, showing essentially an inverse linear relationship with polymer molecular weight.
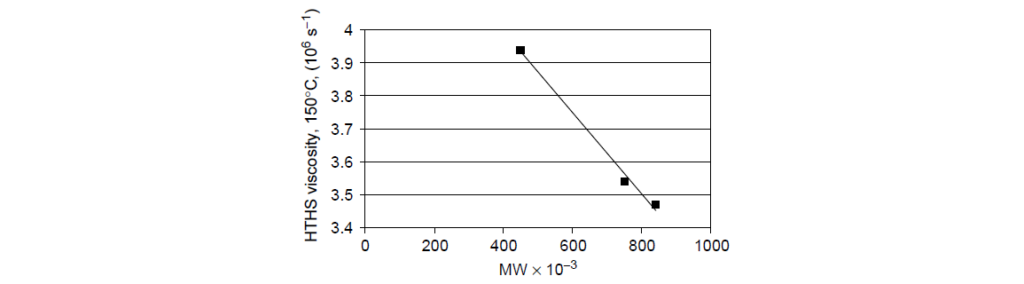
Permanent viscosity loss:
Permanent viscosity loss through mechanical degradation occurs when very high shear stresses, perhaps coupled with turbulent flow, lead to extreme polymer coil distortion and concentrate enough vibrational energy to cause polymer chain rupture. Cavitation probably also plays an important role by producing intense velocity gradients. Polymer chain rupture occurs through homolytic cleavage of a carbon–carbon bond, statistically near the middle of the polymer chain. The cleavage produces two molecules each having approximately half the molecular weight of the original molecule.
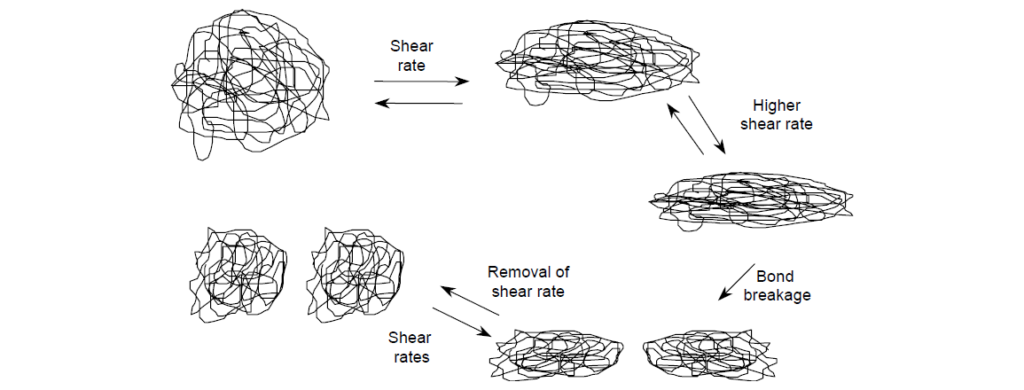
The total hydrodynamic volume of the two smaller molecules is less than that of the single parent molecule, resulting in lower viscosity contribution. Since the bond scission is not reversible, the viscosity loss is permanent. Higher-molecular-weight polymers are more susceptible to distortion and mechanical degradation, whereas polymers of sufficiently low molecular weight may not even undergo permanent shearing. The sheared polymer molecules are typically of sufficiently lower molecular weight that are not normally susceptible to further degradation. Thus, the degradation process is self-limiting under any given intensity of shearing.
As with shear-thinning phenomenon, PMAs of sufficient molecular weight are subject to mechanically induced permanent loss of viscosity, again, the amount of viscosity loss for linear PMA is a reasonably straightforward function of molecular weight. Molecular weight distribution plays a secondary role. If the molecular weight distribution were skewed to larger fractions of high molecular weight polymer, then the loss of viscosity would be greater than that of a polymer of similar average molecular weight but with a Gaussian distribution. It should also be noted that different applications can create very different shear stresses, and because of this, viscosity losses of polymers of a given molecular weight may vary by application. The net result is that viscosity loss to a first approximation is directly related to molecular weight and the amount of shearing force in a given application or laboratory bench test. The relationship of shear stability index to polymer molecular weight and shearing severity by application for a set of PMA VIIs are:
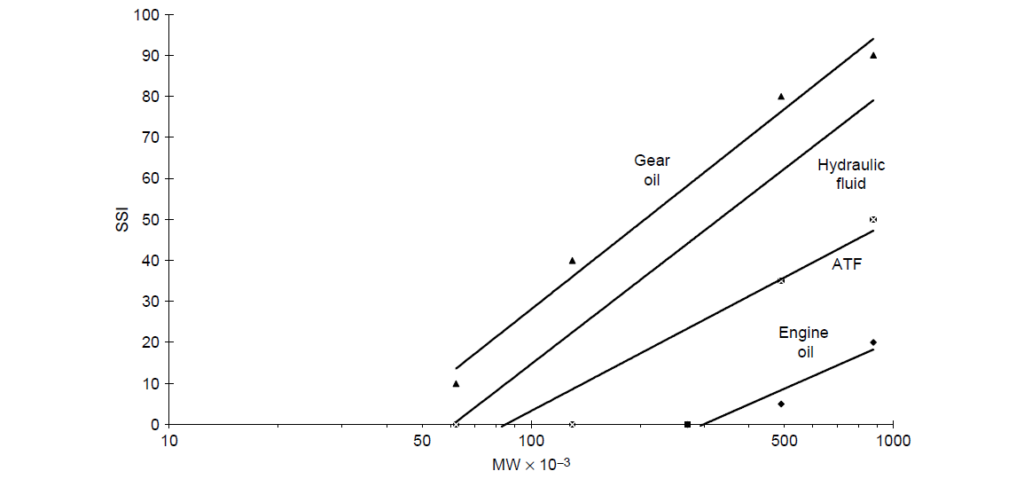
2. Olefin Copolymers [OCPs]:
Olefin copolymer (OCP) viscosity modifiers are oil-soluble copolymers comprising ethylene and propylene.

Classes of Olefin Copolymers
OCPs are marketed as either solids or liquid concentrates. The physical state of the solids depends on several factors, primarily on the ethylene/propylene (E/P) mass ratio. When E/P is in the 45/55–55/45 range, the material is amorphous and cold flows at room temperature. When E/P is higher than 60/40, the copolymer becomes semicrystalline in nature and does not cold flow under ambient conditions.
Liquid concentrates of OCP in mineral oil contain enough rubber to raise the kinematic viscosity (KV) to 500–1500 cSt (mPa s) range at 100°C.
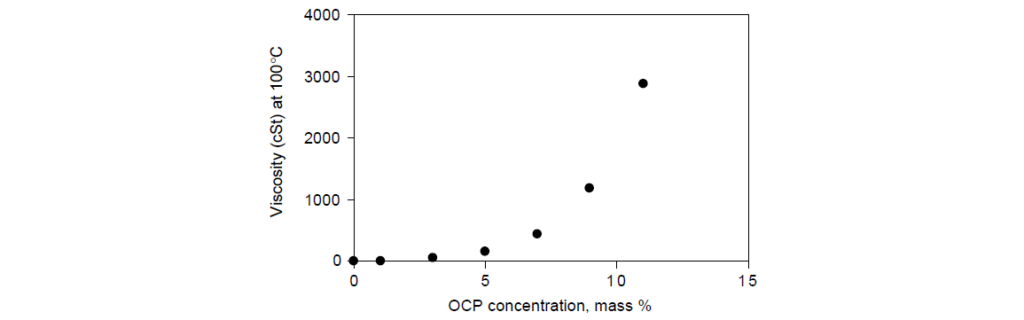
Shear stability is another parameter by which OCP viscosity modifiers are categorized. The higher the molecular weight of a polymer, the more prone it is to mechanical degradation when elongational forces are imposed by the fluid flow field.
Properties and Performance Characteristics
1. Effect of E/P Ratio on Physical Properties:
The comonomer composition of E/P copolymers has a profound influence on the physical properties of the rubber. As the ratio of E/P increases, the fraction of ethylene–ethylene (EE) sequences (dyads) rises. Thus, the average length of contiguous ethylene increases with ethylene content. Above ∼60 wt% ethylene, these sequences become long enough to crystallize, as measured by differential scanning calorimetry.
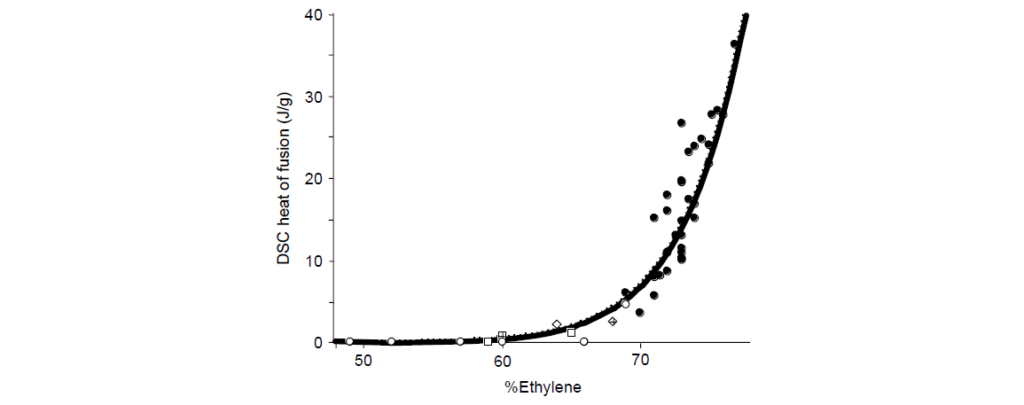
The relatively short ethylene sequences of low-crystallinity (<25%) samples are capable of crystallizing into fringed micelle or short-bundled structures. Higher-order morphologies such as lamellae or spherulites are not observed.
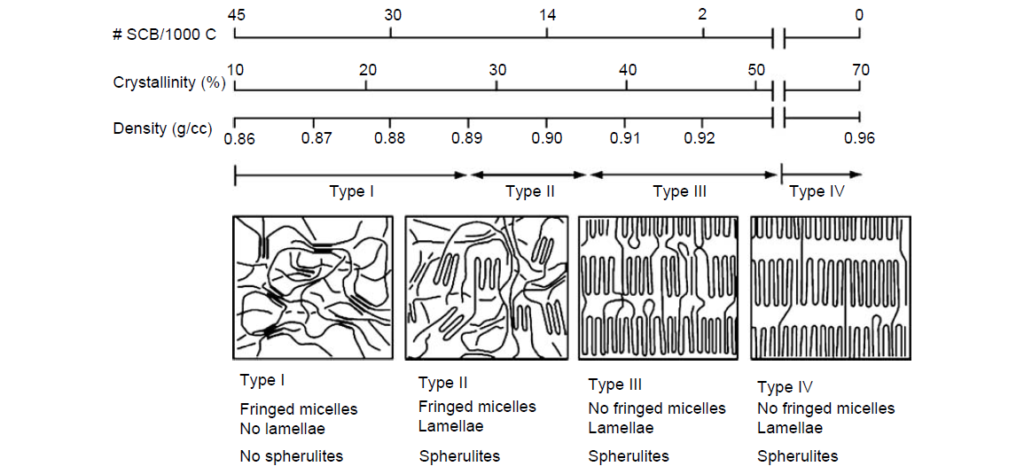
Therefore, the physical properties of semicrystalline OCPs fall in between those of polyethylene and amorphous EP rubber. Polyethylene is a rigid, high-modulus solid at room temperature. Amorphous E/P rubber is a relatively soft material under ambient conditions, which, cold, flows and exhibits a tacky feel. The degree of tack is inversely proportional to its molecular weight and can be reduced by the incorporation of long-chain branching.
2. Effect of Copolymer Composition on Rheological Properties in Solution:
I. Low-Temperature Rheology:
Intrinsic viscosity, a measure of polymer coil size in dilute solution, is fairly insensitive to temperature for non-crystalline OCPs. Semi-crystalline copolymers undergo a precipitous drop in [η] as temperature drops below ∼10°C. In this region, the polymer begins to crystallize, forming intermolecular associations that effectively cause the molecule to shrink in on itself, yet remain sufficiently solvated to remain suspended in oil solution.
The viscosity of a dilute polymer solution often follows the Huggins equation:
ηsp/c = η + k’ η2 c
| Where; | ηsp: | Specific Viscosity (η – η0)/η0 | η: | Solution Viscosity | k’: | Constant |
| η0: | Solvent Viscosity | c: | Polymer Concentration |
Thus, for a specific lubricant composition, c and η0 are fixed, and the temperature dependence of the solution viscosity is directly related to that of the intrinsic viscosity.
Low-temperature viscosity is an important rheological feature of automotive lubricants. For the vehicle to start in cold weather, the lubricant viscosity in the bearings should be below a critical value as determined by low-temperature engine startability experiments. The mechanism of intermolecular crystallization, which leads to molecular size contraction in solution, affords high ethylene OCP viscosity modifiers the opportunity to contribute less to viscosity at low temperatures than noncrystalline or amorphous copolymers of similar molecular weight. For this reason, the class of EP copolymers having ethylene content greater than ∼60 wt% are often called low-temperature OCPs or LTOCPs. A rheological comparison of two LTOCPs and one conventional OCP viscosity modifier in several SAE 5W-30 oil formulations is:
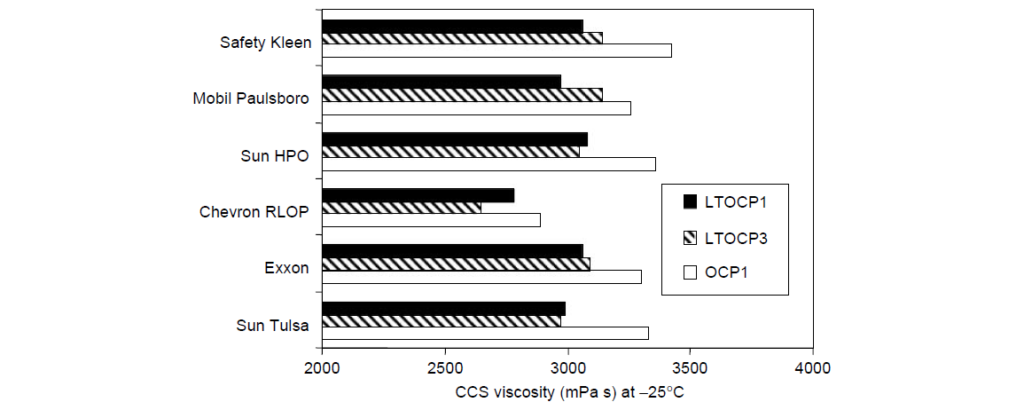
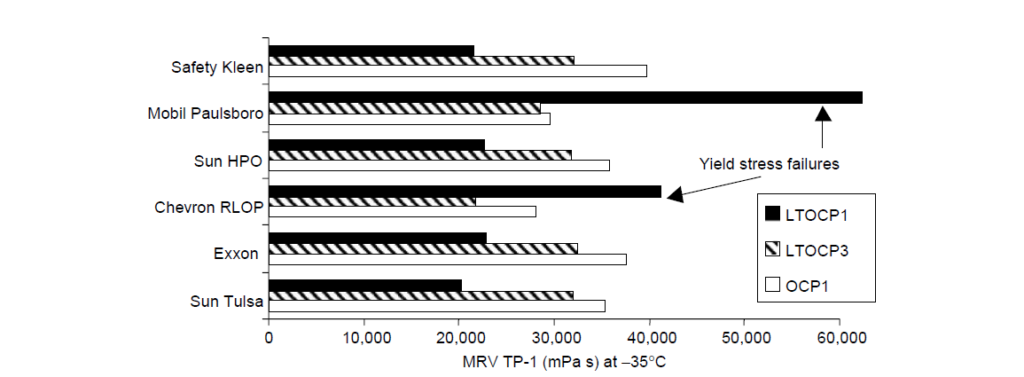
II. High-Temperature Rheology:
Copolymer composition has less influence on high-temperature rheological behavior than it has at low temperatures, partly because lightly crystalline OCPs have melting points well below 100°C . Since both high-temperature KV and high-temperature high-shear rate viscosity (HTHS) are used to classify multigrade engine oils, it is important to understand how copolymer composition and molecular weight influence these key parameters.
a. Kinematic Viscosity (KV):
Thickening efficiency is the amount of polymer necessary to increase the kinematic viscosity of a reference oil to a certain value. It is important to quantify the effects of molecular weight, molecular weight distribution, and branching on thickening efficiency.
For polymers of equal molecular weight, thickening efficiency increases with ethylene content.
Intrinsic viscosity (η) as a function of weight average molecular weight (M) is: η = K Ma. Assuming a single value for the power law constant a = 0.74, (K) values as a function of ethylene content is as below table. This clearly shows that thickening efficiency can be improved by increasing ethylene concentration.
| Mol% ethylene | K × 104 | Mol% ethylene | K × 104 | Mol% ethylene | K × 104 | Mol% ethylene | K × 104 |
| 5 | 2.020 | 30 | 2.485 | 55 | 3.020 | 80 | 3.645 |
| 10 | 2.115 | 35 | 2.585 | 60 | 3.140 | 85 | 3.790 |
| 15 | 2.205 | 40 | 2.690 | 65 | 3.260 | 90 | 3.940 |
| 20 | 2.295 | 45 | 2.795 | 70 | 3.385 | 95 | 4.240 |
| 25 | 2.390 | 50 | 2.910 | 75 | 3.515 | — | — |
The 80 mol% ethylene copolymer requires less polymer to achieve a target viscosity than a 60 mol% copolymer; therefore the former has a higher thickening efficiency than the latter.
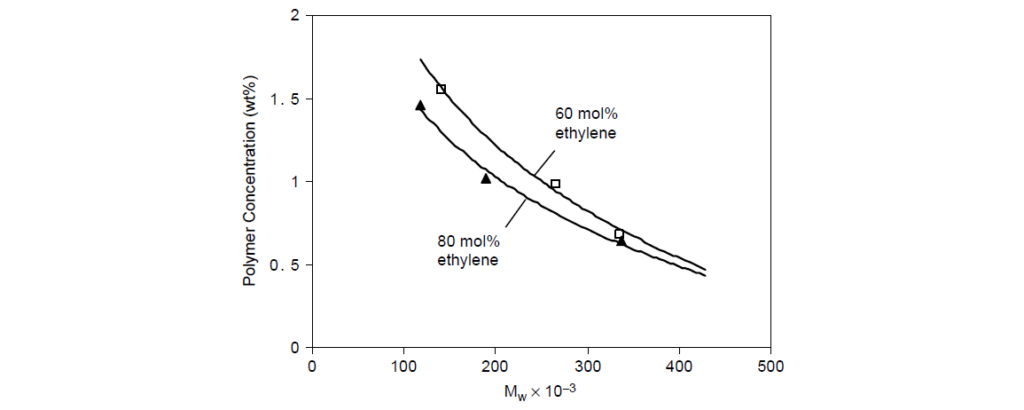
Long-chain branching has a directionally detrimental effect on thickening efficiency for polymers of equal molecular weight.
This is not surprising, since the average chain end-to-end distance of a random coil in solution is controlled, in large part, by the length of the main chain. Branching essentially shortens the chain and lowers its hydrodynamic radius. For example, following thickening efficiency data for two sets of noncrystalline OCP viscosity modifiers, one linear and the other containing 2% branching agent, each set differing only in molecular weight.
| Mw | Linear | Branched |
| 230,000 | 13.50 | 12.03 |
| 180,000 | 11.17 | 10.87 |
Note: Thickening efficiency is defined as the kinematic viscosity (at 100°C) of a 1.0 wt% polymer solution in 6.05 cSt mineral oil.
b. High-Temperature, High-Shear Rate Viscosity (HTHS):
Since concentric journal bearings operate in the hydrodynamic or elastohydrodynamic lubrication regimes, oil film thickness is a critical factor influencing wear. For this reason, SAE J300 specifies a minimum HTHS viscosity for each viscosity grade. HTHS viscosity is measured at very high shear rates (106 s-1) and temperatures (150°C), which is similar to the flow environment in an operating crankshaft bearing at steady state.
At these rates of deformation, most high-molecular-weight polymers will align with the flow field, and a temporary reduction in viscosity is measured. The difference between low-shear rate viscosity and HTHS at 150°C is termed temporary viscosity loss (TVL) or percent temporary viscosity loss (relative to the low-shear rate KV).
As is true for most polymers, TVL is proportional to molecular weight. For polymers of equal weight average molecular weight, those with narrow molecular weight distributions undergo less TVL than those with broad Mw / Mn values.
| Viscosity | Weight Average | Permanent Shear | Capillary Viscosity | HTSH Viscosity | % TVL |
| Modifier | Molecular Weight | Stability Index | @ 150°C | @ 150°C | |
| OCP 1 | 160,000 | 45 | 5.33 | 3.43 | 36 |
| OCP 2 | 80,000 | 30 | 5.33 | 3.77 | 29 |
| OCP 3 | 50,000 | 22 | 5.33 | 3.88 | 27 |
HTHS viscosity can be adjusted by increasing the viscosity of the base oil or by increasing the viscosity modifier concentration. Since the formulation also has to meet KV and CCS viscosity limits, there is often only limited flexibility to adjust HTHS viscosity within the bounds of a given set of base oils and additives.
c. Permanent Shear Stability:
The tendency of an OCP molecule to undergo chain scission when subjected to mechanical forces is dictated by its molecular weight, molecular weight distribution, ethylene content, and degree of long-chain branching. Mechanical forces that break polymer chains into lower-molecular-weight fragments are elongational in nature, causing the molecule to stretch until it can no longer bear the load. This loss in polymer chain length leads to a permanent degradation of lubricant viscosity at all temperatures. In contrast to temporary shear loss, permanent viscosity loss (PVL) represents an irreversible degradation of the lubricant and must be taken into account when designing engine oil for commercial use.
PVL is similar to TVL, except that the viscosity loss is measured by KV before and after shear. Permanent shear stability is more commonly defined by the permanent shear stability index (PSSI) or simply SSI.
PSSI = SSI = 100 x (V0 – Vs) / (V0 – Vb)
| Where, | V0 : | Viscosity of Unsheared Oil |
| Vs : | Viscosity of Sheared Oil | |
| Vb : | Viscosity of Base Fluid (without polymer) |
SSI represents the fraction of viscosity contributed by the viscosity modifier that is lost during shear. SSI is proportional to log10 molecular weight (Mw). Commercial OCP viscosity modifiers have SSI values in the range of 23–55.
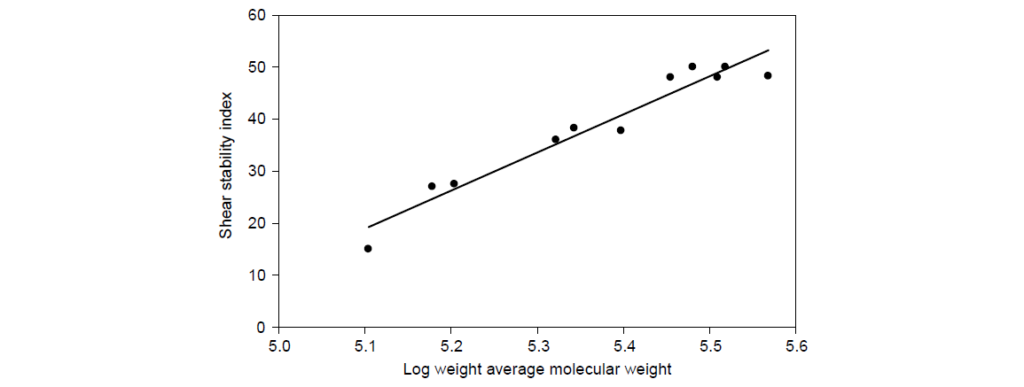
The concept of “stay-in-grade” is generally used to refer to a lubricating oil which maintains its kinematic viscosity within the limits of its original SAE viscosity grade. The problem with viscosity measurements of engine drain oils is that many factors other than permanent polymer shear influence viscosity—such as fuel dilution, oxidation, and soot accumulation. Therefore, it is customary to measure PVL after shear in a laboratory rig, the most common being the Kurt Orbahn test, ASTM D6278.
The concept of “stay-in-grade” is generally used to refer to a lubricating oil which maintains its kinematic viscosity within the limits of its original SAE viscosity grade. The problem with viscosity measurements of engine drain oils is that many factors other than permanent polymer shear influence viscosity—such as fuel dilution, oxidation, and soot accumulation. Therefore, it is customary to measure PVL after shear in a laboratory rig, the most common being the Kurt Orbahn test, ASTM D6278.
The Viscosity Loss Trapezoid (VLT) is a pictorial scheme for mapping the effects of shear rate and PVL on high temperature viscosity. The corners of the trapezoid are defined by viscosity data, and the points are connected by straight lines. The straight lines do not imply that there is a linear relationship between viscosity and shear rate.
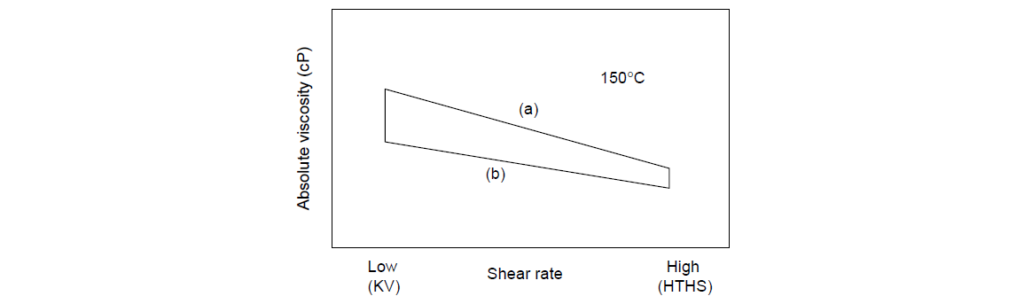
The VLT is a convenient graphical representation of the temporary and permanent shear loss characteristics of polymer-containing oils. Molecular weight degradation causes a permanent loss in both kinematic viscosity and HTHS, but the magnitude of the former is always larger than the latter. The shape of the VLT is the characteristic of polymer chemistry and molecular weight.
It is experimentally observed that the Kurt Orbahn shear test breaks molecules above a threshold molecular size; molecules smaller than the threshold value are resistant to degradation.
3. Comparative Rheological on Performance in Engine Oil:
I. Comparative Study of OCP Viscosity Modifiers in a Fixed SAE 5W-30 Engine Oil Formulation:
Four OCP viscosity modifiers were blended into an SAE 5W-30 engine oil composition consisting of a 95/5 w/w blend of Canadian 100N/250N mineral base stocks, an ILSAC GF-2 quality performance additive, and a polyalkylmethacrylate PPD.
| Viscosity Modifier | SSI | Copolymer Type |
| OCP 1 | 55 | EP, LTOCP |
| OCP 2 | 50 | EPDM, amorphous |
| OCP 3 | 37 | EP, amorphous |
| OCP 4 | 25 | EP, amorphous |
Comparing OCP 1 and OCP 2, the former is a more efficient thickener because it contains no long-chain branching and it has a higher ethylene content. The latter property also manifests itself in lower CCS viscosity. The MRV viscosity is also lower for OCP 1 relative to the other amorphous OCPs, but this is highly dependent on the particular PPD that was chosen for this study. The fact that most of the oils displayed yield stress failures at -35°C shows that the PPD was not optimized for this particular set of components.
As SSI decreases from OCP 1 to OCP 4, the polymer concentration needed to reach a kinematic viscosity target of 10 cSt increases. In other words, polymer thickening efficiency is proportional to shear stability index. Among the noncrystalline OCPs, increasing polymer level causes the CCS viscosity to increase. Since all oils were formulated with the same base oil composition, high- temperature HTHS is relatively constant, independent of OCP type. OCP4, the lowest molecular weight polymer,
should have the lowest degree of Temporary Viscosity Loss, and it indeed has the highest HTHS viscosity of the group.
| OCP 1 | OCP 2 | OCP 3 | OCP 4 | |
| Polymer content (wt%) | 0.58 | 0.71 | 0.73 | 1.05 |
| Kinematic viscosity (cSt) at 100°C | 10.17 | 10.09 | 9.99 | 10.10 |
| Viscosity index | 156 | 160 | 160 | 158 |
| CCS viscosity (cP) at -25°C | 3,080 | 3,280 | 3,510 | 3,760 |
| MRV viscosity (cP) at -30°C | 13,900 | 26,500 | 26,700 | 28,100 |
| MRV viscosity (cP) at -35°C | 40,100 | Yield stress | Yield stress | Yield stress |
| HTHS (cP) | 2.95 | 2.88 | 2.96 | 3.07 |
II. Comparative Study of 37 SSI OCP Viscosity Modifiers in an SAE 15W-40 Engine Oil Formulation:
In this example, the base oil ratio and polymer concentration were adjusted to achieve the following targets: kinematic viscosity = 15.0 cSt and CCS = 3000 cP at -15°C. The base stocks were American Petroleum Institute (API) Group I North American mineral oils, the additive package was of API CH-4 quality level, and the PPD was a styrene ester type that was optimized for these base oils.
| Viscosity Modifier | SSI | Copolymer Type |
| OCP 7 | 37 | EPDM, amorphous |
| OCP 3 | 37 | EP, amorphous |
| OCP 8 | 37 | EPDM, LTOCP |
| OCP 9 | 37 | EP, LTOCP |
LTOCPs inherently lower CCS viscosity contributions permit the greater use of higher- viscosity base oils, which can be beneficial in meeting volatility requirements. The low-temperature MRV performance of OCP9 was far inferior to that of the other copolymers, indicating that the PPD chosen for this particular study was not optimized for OCP9 in these base stocks. Another polyalkymethacrylate PPD was found to bring the MRV viscosity of the OCP9 formulation down to 7,900 and 18,000 cP at -20 and -25°C, respectively.
Again, the higher thickening efficiency of EP copolymers versus EPDMs of similar molecular weight (shear stability) is clearly demonstrated. Another feature worth noting is that increasing base oil viscosity can nudge HTHS viscosity upward (compare OCP7 with OCP8 or OCP3 with OCP9).
| OCP 7 | OCP 3 | OCP 8 | OCP 9 | |
| Polymer content (wt%) | 0.95 | 0.85 | 0.85 | 0.64 |
| 150N base oil percentage | 76 | 76 | 70 | 70 |
| 600N base oil percentage | 24 | 24 | 30 | 30 |
| Kinematic viscosity (cSt) at 100°C | 14.05 | 14.97 | 15.25 | 15.12 |
| Viscosity index | 141 | 140 | 140 | 135 |
| CCS viscosity (cP) at -15°C | 3,080 | 3,040 | 3,070 | 3,010 |
| MRV viscosity (cP) at -20°C | 10,000 | 9,900 | 8,800 | Solid |
| MRV viscosity (cP) at -20°C | 20,500 | 18,600 | 18,300 | Solid |
| HTHS (cP) | 4.17 | 4.38 | 4.25 | 4.42 |
4. Field Performance Data:
Multigrade lubricants containing EP viscosity modifiers have been tested in passenger car and heavy-duty truck engines for over three decades, but very few studies devoted to VM effects on engine cleanliness and wear have been published. It is generally believed that adding a polymer to the engine lubricant will have a detrimental effect on engine varnish, sludge, and piston ring-pack deposits, but the performance additive can be formulated to compensate for these effects.
Kleiser ran a taxicab fleet test designed to compare the performance of a nonfunctionalized OCP viscosity modifier with a highly dispersant-functionalized OCP (HDOCP) as well as to test other oil formulation effects. They were surprised to find that:
SAE 5W-30 oil containing a higher concentration of non-DOCP showed statistically better engine deposit control when compared to a similar SAE 15W-40 oil with lower polymer content.
They also observed significant improvements in sludge and varnish ratings attributed to the use of HDOCP. Others have also reported that DOCPs can be beneficial in preventing buildup of deposits in laboratory engines such as the Sequence VE, VD, and Caterpillar 1H2/1G2 tests. These authors found that engine oils containing certain DOCPs need less ashless dispersant to achieve an acceptable level of engine cleanliness than NOCPs. The actual level of deposit prevention is highly influenced by the functionalization chemistry as well as the number of substituents per 1000 backbone carbon atoms.
| Sample | Nitrogen (wt%) | TGF | WTD |
| OCP | 0.00 | — | > 800 |
| MFOCP5 | 0.30 | 46 | 244 |
| MFOCP6 | 0.50 | 39 | 173 |
| MFOCP7 | 0.70 | 47 | 149 |
| MFOCP6a | 0.26 | 28 | 156 |
| MFOCP2a | 0.28 | 11 | 139 |

Facebook Comments