Endless Improvements
العربية
Thermal Degradation
If the system temperature exceeds 200oC, the typical thermal stability point of lubricant, thermal cracking will occur. The excessive heat and cracking involved causes:
Molecule shearing: they volatize; leaving no deposits.
Polymerization: they condense, causing dehydrogenation and producing lacquer and coke deposits.
Decreasing the viscosity: it is the primary indicator of thermal degradation, compared to the increased viscosity occurring during oxidation.
Overheating Sources:
1. Lubricant comes in contact with hot surfaces.
2. Insufficient circulation of the oil.
3. Micro-dieseling.
It is a sudden increase in temperature associated with the adiabatic compression of entrained air bubbles in pressurized lubrication environments.
Mechanism: If the gas bubbles pass through a higher pressure zone, they will violently collapse. The compression of these bubble in the pressurized side of the pump is adiabatic.
For Example: consider a hydraulic system with a suction-side air leak that lets the bubbles at a atmospheric pressure and 37 oC , and then pressurizes the fluid to 125 bar. The temperature in this example, which is typical of a hydraulic system with an air leak, would be just more than 1,100oC.
Chemistry of Thermal Cracking:
Thermal cracking is taking place in three steps:
1. Chain Initiation Step: Homolytic bond cleavage
Homolytic bond cleavage
R-R  R
R + R
+ R
2. Chain Propagation Step:
At High Pressure: Hydrogen abstraction
R + R1-H
+ R1-H  R-H + R1
R-H + R1
At Low Pressure: 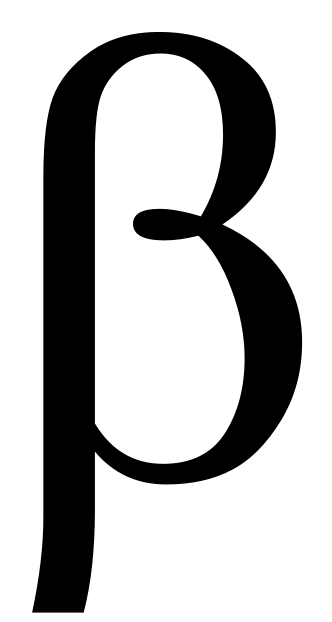 Scission
Scission

3. Chain Termination Step:
Radical Combination:
R2 + R3
+ R3
 R – R
R – R
Disproportionation
2 R2
 R – H + R-CH=CH-R
R – H + R-CH=CH-R
Thermal Failure Indicators:
Lubricant Evaporation: Decreasing in Viscosity and Flash Point.
Insoluble long-chain molecules: Coke Forming.

Facebook Comments