Endless Improvements
العربية
Additives – Detergents
One of the most critical properties of the automotive lubricants, especially engine oils, is their ability to suspend undesirable products from thermal and oxidative degradation of the lubricant.
Such products form when the by-products of fuel combustion go past piston rings into the lubricant and initiate lubricant oxidation. The resulting oxidation products are thermally labile and decompose to highly polar materials with a tendency to separate from the bulk lubricant and form surface deposits and clog small openings. The former will lead to malfunctioning of the closely fitted surfaces, such as those between pistons and cylinder walls, and the latter will impair oil flow to parts needing lubrication.
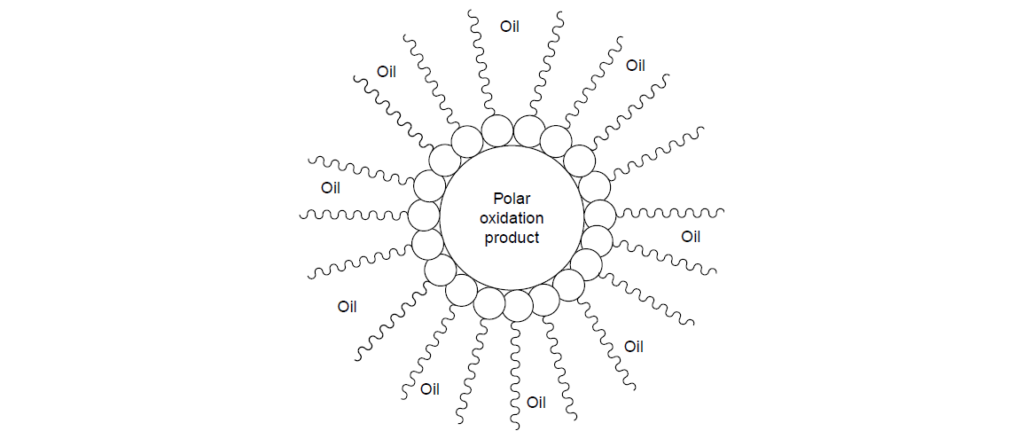
The separation tendency of these products relates to their high polar to nonpoplar ratio, which makes them less soluble in largely nonpolar base oil. A lubricant with high-oxidation resistance, due to the quality of the base fluid or the presence of a good oxidation inhibitor additive package, will slow down the formation of these undesirables.
Oxidation inhibitors, detergents, and dispersants make up the general class of additives called stabilizers and deposit control agents. These additives are designed to control deposit formation, either by inhibiting the oxidative breakdown of the lubricant or by suspending the harmful products already formed in the bulk lubricant.
Detergent Mechanism
Detergents contain reserve base that will neutralize the acids to form salts. Although this decreases the corrosive tendency of the acids, the solubility of the salts in the bulk lubricant is still low.
The organic portion of the detergent, commonly called soap, has the ability to associate with the salts to keep them suspended in the bulk lubricant. The soap in detergents also has the ability to suspend nonacidic oxygenated products such as alcohols, aldehydes, and resinous oxygenates.
Detergent Types
Detergents are metal salts of organic acids. Detergents are composed of an oil-soluble portion, called the substrate, and a metallic-counterion portion.
The chemical structure of the substrate may vary considerably; however, it is typically an organic acid attached to a hydrocarbon chain. The common substrates in commercial use today include:
Arylsulfonic acids.
Alkylphenols.
Alkenylphosphonic acids.
Alkenylthiophosphonic acids.
Carboxylic acids.
Salicylic acids.
A variety of metallic counterions can be combined with the organic acids to form the detergents. Although a number of metals can be used to make neutral salts (soaps), only a fewer metals have the ability to result in oil-soluble basic detergents. The common metals that can be used for this purpose include: Lithium, sodium, and potassium in group I and magnesium, calcium, strontium, and barium in group II of the periodic table.
The reaction of these acids with inorganic bases, such as metal oxides, metal hydroxides, and metal carbonates, results in the formation of salts:
| A – H | + | M – OH | A – M | + | H2O | |
| Acidic substrate | Metal Hydroxide | Neutral metallic Detergent | Water |
A–M is the neutral salt of an acidic detergent substrate and may also be referred to as the soap or surfactant. In general, the soap is the stoichiometric reaction product of one chemical equivalent of an acid substrate plus one equivalent of metal base.
Basic metal detergents are made in a manner similar to forming the neutral detergent. The metal hydroxides used in these basic detergents are typically divalent in character. For example, calcium, magnesium or barium, have been used.
| A – H | + | HO – M – OH | A – M – OH | + | H2O | |
| Acidic substrate | Divalent Metal Hydroxide | Basic metallic Detergent | Water |
The overbased detergents is a fully oil soluble detergent which contain up to 30 times more metal than the normal neutral detergents. A typical method of preparing overbased detergents is:
| A – H | + | 2x M – OH | Promoter CO2 | A−M . x M2CO3 | + | x H2O |
| Acidic substrate | Metal Hydroxide | Overbased Detergent | Water |
| Sulfonate | Phenate | Carboxylate | |
| Neutral | (RSO3)aM | (RPhO)aM | (RCOO)aM |
| Basic | (RSO3)aM . xMbCO3.yM(OH)c | (RPhO)aM . xMbCO3 . yM(OH)c | (RCOO)aM . xMbCO3 . yM(OH)c |
If the metal M is divalent; a and c = 2 and b = 1
| Neutral | Basic | |
| Sulfonate | (RSO3)aM | (RSO3)aM . xMbCO3.yM(OH)c |
| Phenate | (RPhO)aM | (RPhO)aM . xMbCO3 . yM(OH)c |
| Carboxylate | (RCOO)aM | (RCOO)aM . xMbCO3 . yM(OH)c |
If the metal M is divalent; a and c = 2 and b = 1
Detergent Parameter
Detergents are described chemically in terms of their metal ratio, percent sulfated ash, degree of overbasing or conversion, soap content, and total base number (TBN).
Metal Ratio:
The metal ratio is defined as the total equivalents of metal per equivalent of acid.
Percent Sulfated Ash:
The percent sulfated ash is the ash produced when the detergent is treated with sulfuric acid and burned. All organic material in the detergent burns, leaving behind the metal sulfate ash. Detergents are not the only additives that result in sulfate ash. Other metal-containing additives in the lubricant also contribute toward it.
Because the metal compounds can lead to the formation of the inorganic material (ash) on combustion, a formulator must know the metal content of a formulation to offset any problem that might occur. This is because the lubricant travels past piston rings into areas that experience flame and high temperatures, such as the top land and the groove behind the top ring; it burns to produce ash. Ash is undesired because it is believed to initiate deposit formation. Sulfated ash is one of the methods used to assess the metal content of a lubricant, and the methods to determine this are described in the ASTM Standards D 482 and D 874.
Degree of Overbasing :
The degree of overbasing is the number of equivalents of the metal base per equivalent of the acid substrate. This is usually expressed as conversion, which indicates the amount of inorganic material relative to that of organic material. Conversion is expressed as the number of equivalents of base per equivalent of acid times 100.
Soap Content:
The soap content is the amount of neutral salt as a percent of detergent composition.
Total Base Number – TBN:
The TBN of the detergent reflects its ability to neutralize acids. In additives and formulated lubricants, the TBN is expressed as mg KOH/g of additive. The method to determine base numbers is described in the ASTM Standard D 974.
Sulfonate and phosphonate detergents: The overbased portion of the detergent only possesses the acid-neutralizing ability. The neutral metal sulfonates and phosphonates lack this ability.
Carboxylates, salicylates, and phenates soap: possess the acid-neutralizing ability. This is because, unlike sulfonates and phosphonates that are strong acid–strong base salts. This makes them Lewis bases, hence the acid-neutralizing ability.
Detergent Testing
A variety of proprietary and industry-established tests are used to determine a detergent’s effectiveness in lubricants. It is important to note that technically most of these tests evaluate the combined effectiveness of the detergent and the dispersant. The performance of the individual additive is hard to unravel.
Gasoline Engines:
For gasoline engine oils to be used in North America, these include:
CRC L-38.
TEOST: Thermo-Oxidation Engine Oil Simulation Test.
ASTM Sequence IIIE/IIIF.
ASTM Sequence VE/VG tests.
For European gasoline engines:
ASTM Sequence IIIE/IIIF.
ASTM Sequence VE/VG.
Engine tests Peugeot TU3M High-Temperature Test.
MB M111 Black Sludge Test.
Sulfated Ash.
These tests are part of the ACEA 2002 Standard.
Diesel Engines:
The efficacy of diesel engine oils for North American use is evaluated by the use of both the single-cylinder and the multicylinder engine tests.
Single-cylinder tests include:
Caterpillar 1M-PC. [Part of the API CF, CF-2, and the U.S. Military’s MIL-PRF-2104G specifications].
Caterpillar 1K. [Part of the API CF-4, API CH-4, and API CI-4 standards].
Caterpillar 1N is a part of the API CG-4, API CI-4, and the U.S. Military’s MIL-PRF-2104G specifications.
Caterpillar 1P. [Part of the API CH-4 specification].
Caterpillar 1R. [Part of the API CI-4 specification].
The pass ratings for these tests require meeting the overall appearance of the cylinder and its parts, which are expressed in terms of weighted demerits, percent top groove fill, ring side clearance loss, top land heavy carbon, oil consumption, and stuck rings. All these parameters are related to deposits on the piston and its parts.
Multicylinder tests include:
Detroit Diesel 6V92TA engine test. [Part of the API CF-2 and MIL-PRF-2104G specifications].
Mack M11 engine test [Part of the API CH-4 and CI-4 specifications].
These two tests evaluate a detergent’s ability to prevent deposit-related port plugging and engine sludge, respectively.
For European use, oils must pass the requirements of tests such as:
VW1.6L TC diesel.
XUD11BTE.
VWDI.
MB OM 364 LA.
MB OM 441 LA.
These tests evaluate ring sticking, piston cleanliness, viscosity increase, and filter plugging.

Facebook Comments