Endless Improvements
العربية
Additives – Dispersants
Dispersants differ from detergents in three significant ways:
Dispersants are metal-free, but detergents contain metals. This means that on combustion detergents will lead to ash formation and dispersants will not.
Dispersants have little or no acid-neutralizing ability, but detergents do.
Dispersants are much higher in molecular weight, approximately 4–15 times higher, than the organic portion (soap) of the detergent. Because of this, dispersants are more effective in fulfilling the suspending and cleaning functions than detergents.
The dispersants suspend deposit precursors in oil in various ways. These comprise the following:
Including the undesirable polar species into micelles.
Associating with colloidal particles, thereby preventing them from agglomerating and falling out of solution.
Suspending aggregates in the bulk lubricant, if they are formed.
Modifying soot particles so as to prevent their aggregation. The aggregation will lead to oil thickening, a typical problem in heavy-duty diesel engine oils.
Lowering the surface/interfacial energy of the polar species to prevent their adherence to metal surfaces.
Nature of Deposit and Mode of Their Formation
Gasoline and diesel fuels contain highly branched olefins and aromatics which burns to form peroxides, hydroperoxides, and free radicals. The fuel degradation products go past the piston rings into the lubricant as blow-by and attack largely the hydrocarbon lubricant. The reaction between the contents of the blow-by and the lubricant olefins and aromatics results in the formation of the lubricant-derived peroxides and hydroperoxides that either oxidatively or thermally decompose to form aldehydes, ketones, and carboxylic acids.
The reaction between nitrogen and oxygen to form NOx is more prevalent in diesel engines and gasoline engines that are subjected to severe service, such as long-distance driving for extended periods. The NOx formation initiates when the temperature reaches 137°C. Acids can also result from oxidation of the fuel sulfur.
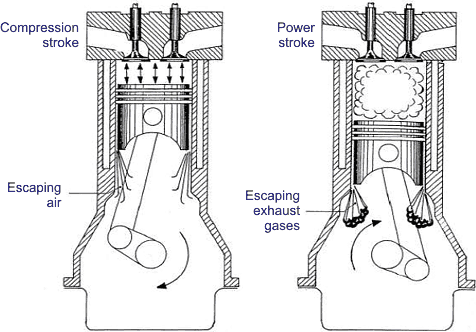
The aldehydes and ketones undergo aldol-type condensation in the presence of bases or acids to form oligomeric or polymeric compounds. These can further oxidize to highly oxygenated hydrocarbons, commonly referred to as oxygenates. The oxygenates are usually of sticky consistency, and the term resin is often used to describe them. Resin is either the basic component in or the precursor to all types of deposits. Common types of deposits include varnish, lacquer, carbon, and sludge. Varnish, lacquer, and carbon occur when resin separates on hot surfaces and dehydrates or polymerizes to make tenacious films. The quantity and the nature of deposits depend on the proximity of the engine parts to the combustion chamber.
Carbon Deposits:
The parts closer to the combustion chamber, such as exhaust valve head and stem that experience approximate temperatures of 630–730°C, will develop carbon deposits. The same is true of the combustion chamber wall, piston crown, top land, and top groove, which are exposed to approximate temperatures of 200–300°C. Carbon deposits are more common in diesel engines than in gasoline engines and result from the burning of the liquid lubricating oil and the high-boiling fractions of the fuel that adhere to hot surfaces.
Lacquer and Varnish:
As we move away from these regions to the low-temperature regions, such as the piston skirt, the deposits are not heavy and form only a thin film. For diesel engine pistons, this type of deposit is referred to as lacquer; for gasoline engine pistons, this type of deposit is called varnish. The difference between lacquer and varnish is that lacquer is lubricant-derived and varnish is largely fuel-derived. In addition, the two differ in their solubility characteristics. That is, lacquer is water soluble and varnish is acetone-soluble. Lacquer usually occurs on piston skirts, on cylinder walls, and in the combustion chamber, whereas varnish occurs on valve lifters, piston rings, piston skirts, valve covers, and positive crankcase ventilation (PCV) valves.
Sludge:
The coolest parts of the engine, such as rocker arm covers, oil screen, and oil pan, that are exposed to temperatures of ≤200°C experience sludge deposits. Sludge can be watery or hard in consistency, depending on the severity of service. If the service is extremely mild and of short duration, as in the case of stop-and-go gasoline engine operation, the sludge is likely to be watery or mayonnaise like. This type of sludge is called low-temperature sludge, which occurs when the ambient temperature is <95°C. The high-temperature sludge is more common in diesel engines and gasoline engines with long, continuous operation. This type of sludge occurs when the ambient temperature is >120°C and is hard in consistency. In the former case, the engine does not get hot enough to expel combustion water, which stays mixed with oil, imparting sludge, a mayonnaise like appearance. In the latter case, however, the ambient temperature is high enough to expel water, thereby resulting in hard sludge. Sludge is common in areas that experience low oil flow, such as crankcase bottoms and rocker boxes.
Soot:
Another component of the combustion effluent that must be considered is soot. Soot not only contributes toward some types of deposits such as carbon and sludge, but it also leads to a viscosity increase. These factors can cause poor lubricant circulation and lubricating film formation, both of which will result in wear and catastrophic failure. Soot is particulate in nature and results from the incomplete combustion of the fuel and of the lubricating oil from the crankcase that might enter the combustion chamber by traveling past the piston rings. Fuel-derived soot is a chronic problem in the case of diesel engines because diesel fuel contains high-boiling components that do not burn
easily. In addition, diesel engine combustion is largely heterogeneous, with poor air–fuel mixing, hence poor combustion. Soot is made of hydrocarbon fragments with some of the hydrogen atoms removed. The particles are charged and hence have the tendency to form aggregates. When aggregates occur on surfaces, such as those of the combustion chamber, soot deposits result. These deposits are soft and flaky in texture. If these occur in oil, lubricant experiences an increase in viscosity. A soot-related viscosity increase usually requires the presence of polar materials in oil that have the ability to associate with soot. These can be additives or polar lubricant oxidation and degradation products. Carbon deposits are lower in carbon content than soot and, in most cases, contain oily material and ash. This makes knowledge of the ash-forming tendency of a lubricant important to a formulator. When soot associates with resin, one gets either resin-coated soot particles or soot-coated resin particles. The first type of particles results when resin is in excess, and the second type results when soot is in excess. The amount of soot in resin determines the color of the deposits: the higher the soot, the darker the deposits. Sludge results when resin, soot, oil, and water mix.
Deposit Control by Dispersants
Fuel and lubricant oxidation and degradation products are of low lubricant (hydrocarbon) solubility, with a propensity to separate on surfaces. The separation tendency of these materials is a consequence of their particle size. Small particles are more likely to stay in oil than large particles. Therefore, resin and soot particles, which are the two essential components of all deposit-forming species, must grow in size through agglomeration before separation. Growth occurs either because of dipolar interactions, as is the case in resin molecules, or because of adsorbed polar impurities such as water and oxygen, as is the case in soot particles. Alternatively, soot particles are caught in the sticky resin.
Dispersants interfere in agglomeration by associating with individual resin and soot particles. The particles with associated dispersant molecules are unable to coalesce because of either steric factors or electrostatic factors. Dispersants consist of a polar group, usually oxygen- or nitrogen-based, and a large nonpolar group. The polar group associates with the polar particles, and the nonpolar group keeps such particles suspended in the bulk lubricant. Neutral detergents, or soaps, operate by an analogous mechanism.
Desirable Dispersant Properties
Dispersing soot and deposits is clearly the primary function of a dispersant. Dispersants need other properties to perform effectively. These include thermal and oxidative stability and good low-temperature properties.
If a dispersant has poor thermal stability, it will break down, thereby losing its ability to associate with and suspend potentially harmful products.
Poor oxidative stability translates into the dispersant molecule contributing itself toward deposit formation.
Good low-temperature properties of a lubricant are desired for many reasons: ease of cold cranking, good lubricant circulation, and fuel economy.
Dispersant Strucrure
A dispersant molecule consists of three distinct structural features: a hydrocarbon group, a polar group, and a connecting group or a link.
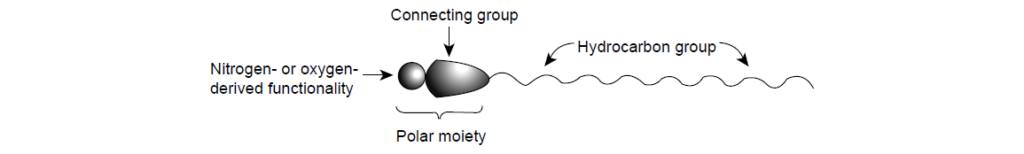
The hydrocarbon group:
The hydrocarbon group is polymeric in nature, and depending on its molecular weight, dispersants can be classified into:
1. Polymeric Dispersants:
Polyisobutylene is the most common source of the hydrocarbon group in polymeric dispersants. It is manufactured through acid-catalyzed polymerization of isobutylene.
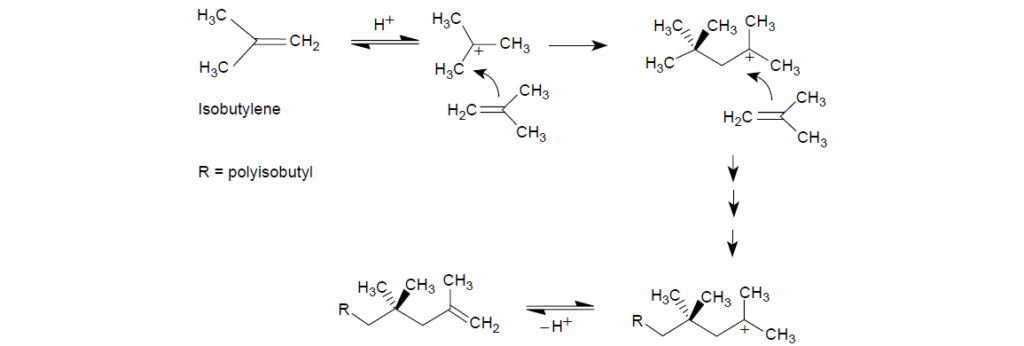
Polyisobutylene is shown as a mixture of various isomers. Polyisobutylenes of structures I and II result from the loss of a proton from carbon 1 and carbon 3 of the intermediate of structure V. Polyisobutylenes of structures III and IV result from the rearrangement of the initially formed carbocation. The reactivity of these olefi ns toward phenol and maleic anhydride varies. In general, the more substituted the olefin, the lower the reactivity, which is a consequence of the steric factors. Similarly, the larger the size of the polyisobutyl pendant group, that is, the higher the molecular weight, the lower the reactivity.
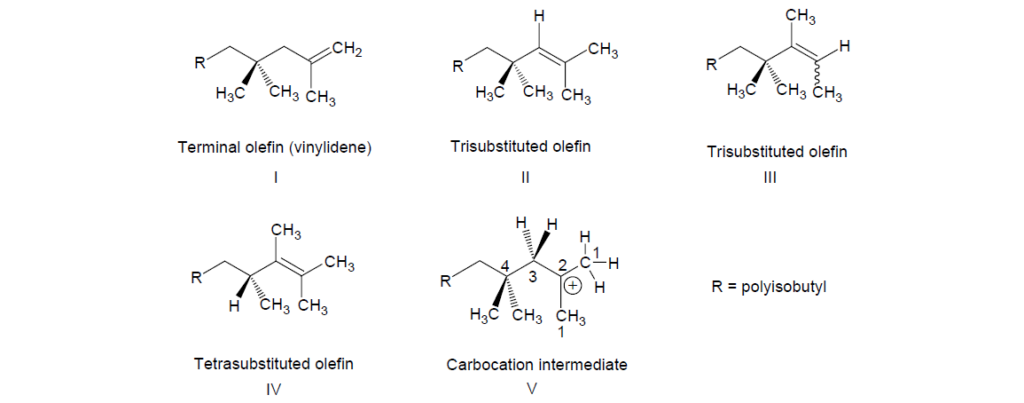
The number average molecular weight (Mn) of polyisobutylene ranges between 500 and 3000. In addition to Mn, other polyisobutylene parameters, such as molecular weight distribution and the length and degree of branching, are also important in determining the overall effectiveness of a dispersant.
2. Dispersant polymer: Molecular Weight of 25,000 and higher.
Dispersant polymers, also called dispersant viscosity modifiers (DVMs) and dispersant viscosity index improvers (DVIIs), are derived from hydrocarbon polymers of molecular weights between 25,000 and 500,000. Polymer substrates used to make DVMs include high-molecular-weight olefin copolymers (OCPs), such as ethylene–propylene copolymers (EPRs), ethylene–propylene–diene copolymers (EPDMs), polymethacrylates (PMAs), styrene–diene rubbers (SDRs) of both linear and star configurations, and styrene–ester polymers (SEs).
The Connecting Group:
Succinimide, phenol, and phosphonate are the common connecting groups used to make dispersants. Of these, succinimide and phenol are the most prevalent. Succinimide group results when a cyclic carboxylic acid anhydride is reacted with a primary amino group. Alkenylsuccinic anhydride is the precursor for introducing the succinimide connecting group in dispersants. Alkenylsuccinic anhydride is synthesized by reacting an olefin, such as polyisobutylene, with maleic anhydride.
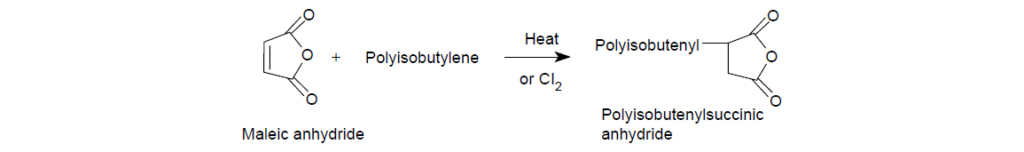
The Polar Moiety:
The two common polar moieties in dispersants are based on polyamines and polyhydric alcohols.
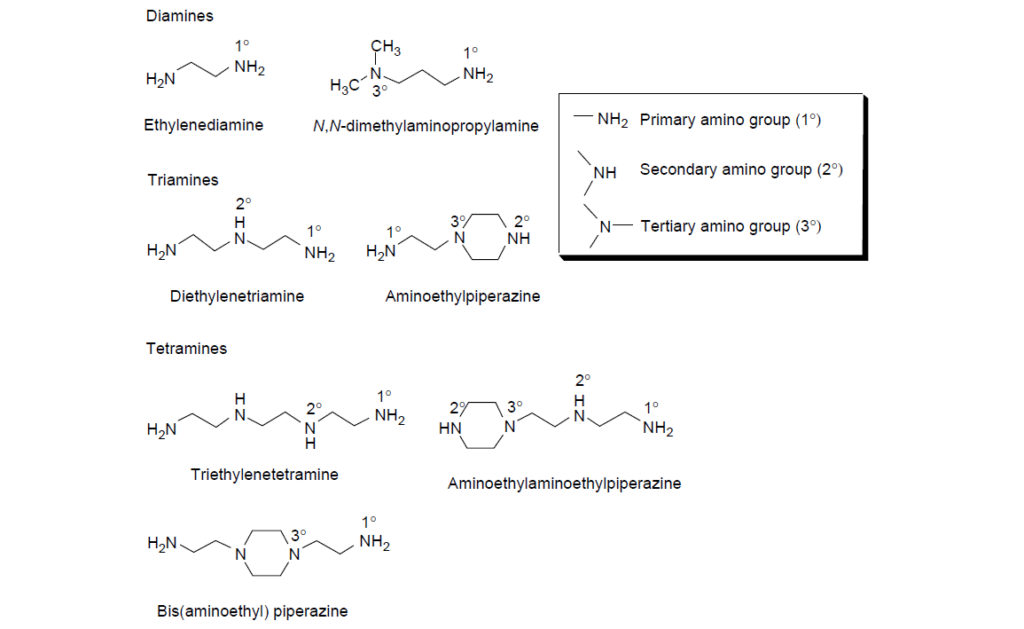
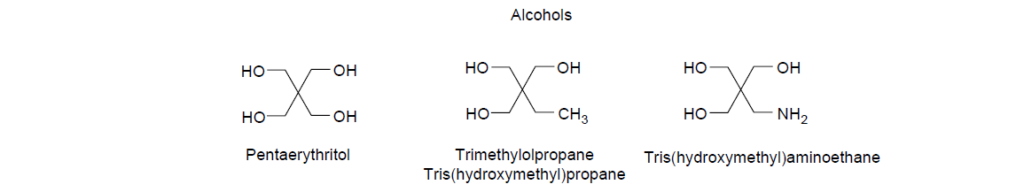
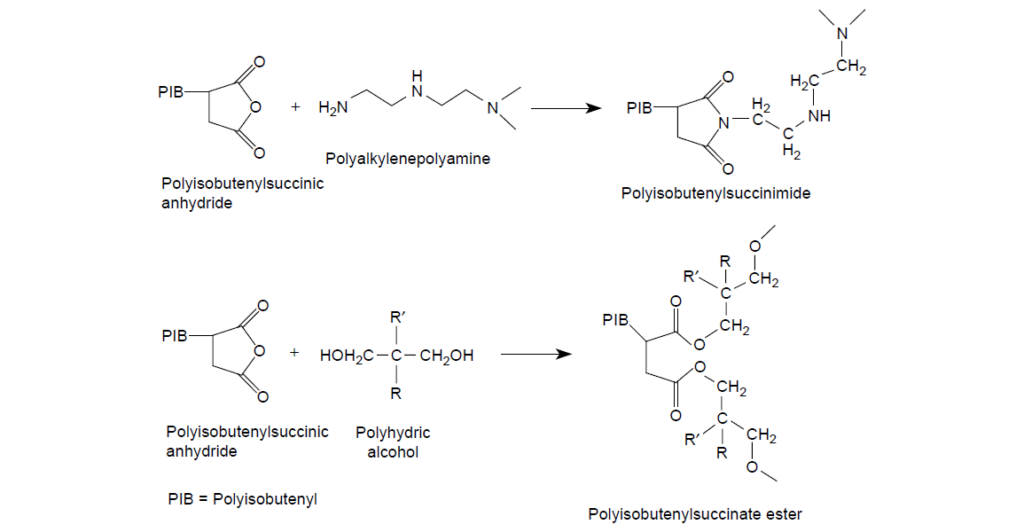
Imide and ester dispersants are made by reacting polyamines and polyhydric alcohols with alkenylsuccinic anhydrides. The reaction typically requires a reaction temperature between 130 and 200°C to remove the resulting water and complete the reaction
Performance Testing
Additive manufacturers use various laboratory screen tests and engine tests to evaluate a dispersant’s effectiveness. Many of the screen tests are proprietary, but all are developed around evaluating performance in terms of a dispersant’s ability to disperse lamp black or used engine oil sludge. The laboratory engine tests are industry-required tests and include both gasoline engine and diesel engine tests.
| Engine Test | Engine Type | Evaluation Criteria |
| CRC L-38 | CLR single-cylinder engine | Bearing corrosion, sludge, varnish, oil oxidation, and viscosity change |
| ASTM sequence IIIE | 1987 Buick V6 engine | Sludge, varnish, wear, and viscosity change |
| ASTM sequence IIIF | 1996 Buick V6 engine | Sludge, varnish, wear, and viscosity change |
| ASTM sequence VE | Ford Dual-Plug head four-cylinder engine | Sludge, varnish, and wear |
| ASTM sequence VG | Ford V8 engine | Sludge, varnish, and wear |
| TEOST | Bench test | Thermal and oxidative stability |
| High-temperature deposit test | Bench test | High-temperature deposits |
| Engine Test | Engine Type | Evaluation Criteria |
| Caterpillar 1K | Caterpillar single-cylinder engine | Piston deposits and oil consumption |
| Caterpillar 1M-PC | Caterpillar single-cylinder engine | Piston deposits and oil consumption |
| Caterpillar 1N | Caterpillar single-cylinder engine | Piston deposits and oil consumption |
| Caterpillar 1P | Caterpillar single-cylinder engine | Piston deposits and oil consumption |
| Mack T-6 | Multicylinder engine | Piston deposits, wear, oil consumption, and oil thickening |
| Mack T-7 | Multicylinder engine | Oil thickening |
| Mack T-8 | Multicylinder engine | Oil thickening |
| Mack T-9 | Multicylinder engine | Soot thickening |
| Engine Test | Engine Type | Evaluation Criteria |
| ASTM sequence IIIE | Six-cylinder engine | High-temperature oxidation (sludge, varnish, wear, and viscosity increase) |
| ASTM sequence VE | Four-cylinder engine | Low-temperature sludge, varnish, and wear |
| Peugeot TU-3M high temp. | Four-cylinder single-point injection engine | Piston deposits, ring sticking, viscosity increase |
| M-B M111 black sludge | Four-cylinder multipoint injection engine | Engine sludge and cam wear |
| VW 1302 | Four-cylinder carbureted engine | Piston deposits, varnish, wear, and oil consumption |
| VW T-4 | Four-cylinder multipoint injection engine | Extended drain capability |
| Engine Test | Engine Type | Evaluation Criteria |
| VW 1.6TC diesel intercooler | Four-cylinder engine | Piston deposits, varnish, and ring sticking |
| VW D1 | Four-cylinder direct-injection engine | Piston deposits, viscosity increase, and ring sticking |
| Peugeot XUD11ATE | Four-cylinder indirect-injection engine | Piston deposits and viscosity increase |
| Peugeot XUD11BTE | Four-cylinder indirect-injection engine | Piston deposits and viscosity increase |
| M-B OM 602A | Five-cylinder indirect-injection engine | Engine wear and cleanliness |
| M-B OM 364A/LA | Four-cylinder direct-injection engine | Bore polishing, piston deposits, varnish, sludge, wear, and oil consumption |
| M-B OM 441LA | Six-cylinder direct-injection engine | Piston deposits, bore polishing, oil consumption, valve train condition, and turbo deposit |
| MAN 5305 | Single-cylinder engine | Piston deposits, bore polishing, and oil consumption |
| Mack T-8 | Multicylinder engine | Soot-related oil thickening |

Facebook Comments