Endless Improvements
العربية
Additives – Antiwear and Extreme Pressure
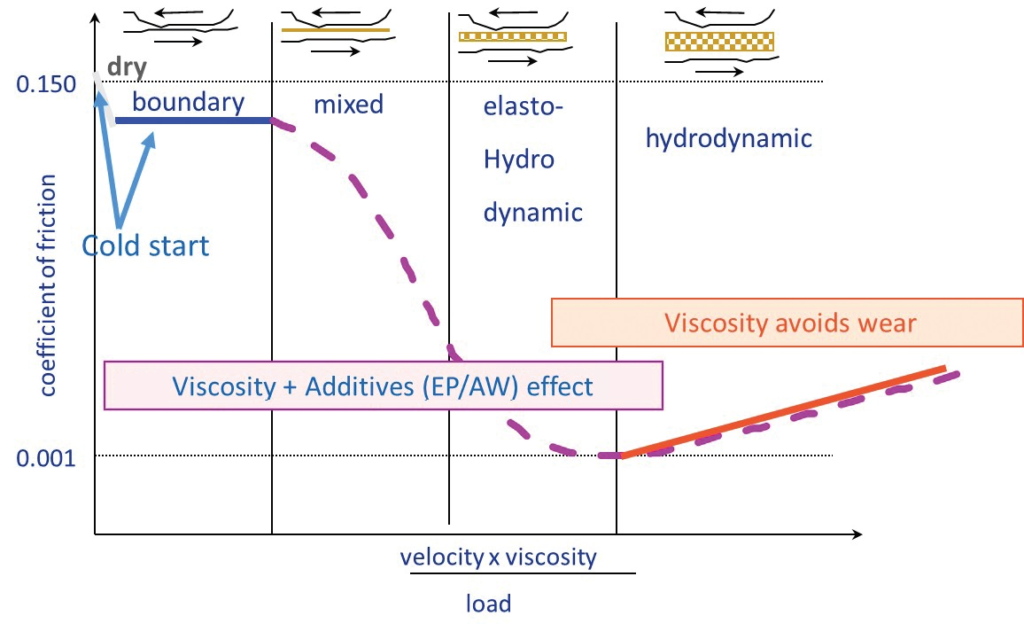
To optimize the balance between low wear and low friction, machine designers specify a lubricant with a viscosity sufficient to generate hydrodynamic oil film that separates the machine’s interacting surfaces, but not too high to induce excessive viscous drag loss.
In practice, many factors conspire to reduce oil film thickness below the optimum; such as:
The various contact types in a machine.
The incidence of operating conditions beyond the design range.
The pressure to improve efficiency by reducing oil viscosity.
The asperities on the interacting surfaces start to interact with one another, initially through micro-elastohydrodynamic lubrication (EHL) films, and at the end through direct surface contact, resulting in increased friction and the likelihood of surface damage. Antiwear and extreme pressure additives are added to lubricating oils to decrease wear and prevent seizure under such conditions Boundary Lubrication (BL).
Chemically;
AW/EP additives are polar and attached to frictional metal surfaces. They must use elements that can form iron compounds for proper reaction with the metal surface.
The heat from metal-to-metal contact activates these additives to form a film that minimizes wear.
This film can withstand compression and prevents the metal surfaces from making contact because the film has lower shear strength than the metal.
The distinction between antiwear and EP additives is not clear-cut. Some are classed as antiwear in one application and EP in another, and some have both antiwear and EP properties.
Antiwear additives are best suited for lubricants that operate under mild conditions with low loads and high speeds and are made to reduce the rate of continuous and moderate wear. Applications of Antiwear additives are widespread and are often used with hydraulic oils, engine oils, gear oils, automatic transmission fluid, and some greases.
Extreme Pressure additives are relegated for use under heavier loads at high temperatures and low speeds. The rate of EP film formation are higher and the film itself is tougher. Lubricants that have EP additives are crucial for the prevention of catastrophic failure or seizing of the application. Extreme Pressure additives are used in more niche applications, which usually include transmission fluids and non-worm gear oils.
Mechanisms of Film Formation
I. Formation of a layer of molecules adsorbed by van der Waals forces:
To form such layers a molecule must have a polar end which attaches to the metal and a nonpolar end which points out into the oil solution. These layers are a shear strength layer which allows motion without high friction and can also reduce wear. The temperature/load range over which they are effective depends upon their individual structure.

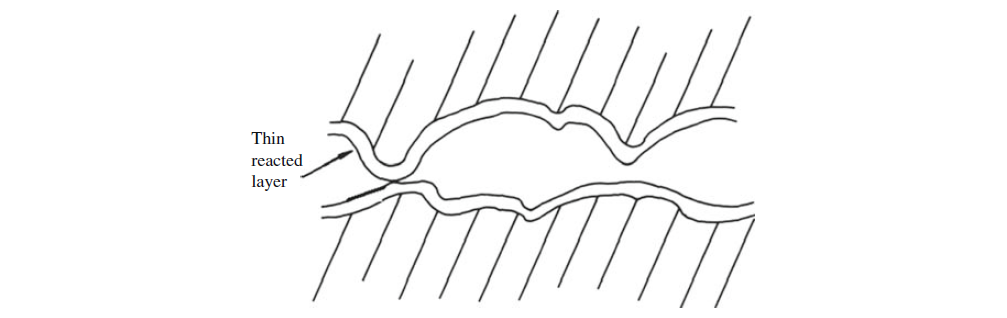
II. Thin reacted layer plus smoothing:
Oil components chemically attack exposed clean metal surfaces to give a thin, chemically altered layer, e.g. a soap. Chemical film formation does not occur to any appreciable depth and abrasion plus this attack smoothes asperities. This fact, together with the low shear strength of the film, leads to reduction of adhesion and ploughing. Moderate temperatures are often required for the formation of these films.
III. Thick reacted inorganic layers:
Reaction between active oil components and metal surfaces forms thick, reacted, low-shear-strength inorganic films such as sulphides on the sliding surfaces. This surface layer limits adhesion but must be reformed as quickly as is practicable or scuffing will occur. These films are usually formed only at high temperature and loads.

Physical Chemical Processes for the Formation of Surface Films
Formation of Surface Films:
The process of surface film formation by additives involves a series of steps, described as:
Interactions between permanent dipoles in the molecule and fluctuating dipoles on the surface.
Residence at the surface.
Desorption back into solution, or chemical reaction between the adsorbed species and the surface.
Diffusion of species to the surface.
Dependent upon temperature and additive concentrations, any of the above can be the rate-limiting step controlling film formation. The issue is further complicated by the temperature-variable influence of interactions of molecules in solution. These can influence the equilibrium between surface and solution by influencing the flux of molecules/unit time impinging upon the surface and the probability of reaction at the surface. To a reasonable approximation, the kinetics of these processes can be described by the Langmuir isotherm
| dθ | = | ka [A] (1 − θ) − kd(θ) |
| dt |
| Where; | θ: | surface coverage. | ka: | rate constants for adsorption. | [A]: | concentration of additive in solution |
| t: | time | kd: | rate constants for desorption. |
Equations of this type can describe both the ‘physical’ dipole/dipole interactions and the subsequent ‘desorption’ from the film. Since ka and kd have different temperature coefficients, increasing temperature can lead to either increased, decreased or unchanged surface coverage. Provided that a critical minimum surface is maintained, wear and friction can be controlled. But once θ falls below this critical value, believed to be approx 0.5, friction and wear will rise.
Influence of Base Stock:
The model for film formation described above suggests that interactions between base stock and film-forming additives, which retard their adsorption, will have a detrimental influence on wear and friction. For instance, highly napthenic base stocks are good solvents for polar species whilst paraffinic base stocks are relatively poor solvents for polar species and will therefore enhance film-forming activity.
Influence of Metal Surfaces:
The mechanisms of film formation previously described involve both physical and chemical processes. It follows that factors favorable to film formation can influence friction and wear. Coefficients of friction were shown to vary from 0.04 for a reactive metal such as zinc, lubricated with 1% lauric acid, to 0.55 for an inert metal, silver, with the same lubricant. These factors include strong dipole interactions or strong hydrogen bonding which aid physical adsorption and the ease of chemical reaction from this adsorbed layer.
Surfaces generated under rubbing conditions are more reactive towards additives than are static surfaces. In part this is due to the presence of oxide films on static surfaces which are removed by sliding contact and the fresh ‘nascent’ metal surface has a higher reactivity than its oxidized predecessor. In addition, rubbing surfaces generate low energy, 1–3 eV, electrons or exoelectrons spontaneously, which can promote chemical reactions, i.e. polymerization.
Corrosive/Adhesive Balance:
Wear control by reactive film formation involves a corrosive wear process to limit the rate of an adhesive wear process. The protective film is removed during the sliding process and must be replaced in time between successive contacts. If the rate of this corrosive film formation process is faster than required to control adhesion, then material loss, i.e. wear, will occur at an unacceptable rate. This excessive corrosion can occur either because the additive used is excessively reactive at the contact temperatures or because the concentration of the additive in the oils is too high. If the additive is present at too low a concentration to maintain film formation, then excessive adhesive wear will occur. Maintenance of this corrosive adhesive balance is essential.
Antiwear and Extreme Pressure Classes of Compounds
friction and/or wear may be controlled by a variety of classes of compounds which can form surface films. These compounds include:
1. Sulfur Additives
Sulfur-containing additives are used to provide protection against high pressure, metal-to-metal contacts in boundary lubrication. The magnitude of the EP activity is a function of the sulfur content of the additive; high-sulfur-content additives are usually more effective EP agents than are low-sulfur-content additives. The sulfur content of the additive must be balanced against requirements for thermal stability and non-corrosiveness toward copper-containing alloys.
Sulfurization by addition of sulfur compounds [elemental sulfur, hydrogen sulfide, and mercaptans] to unsaturated compounds has been known to the chemical industry for years.
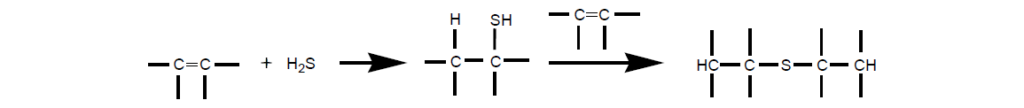
I. Sulfurized Olefins:
Sulfurized olefins are prepared by treating an olefin with a sulfur source under proper reaction conditions. Sulfurized isobutylene (SIB) has been by far the most cost-effective, widely used EP additive in lubricants.
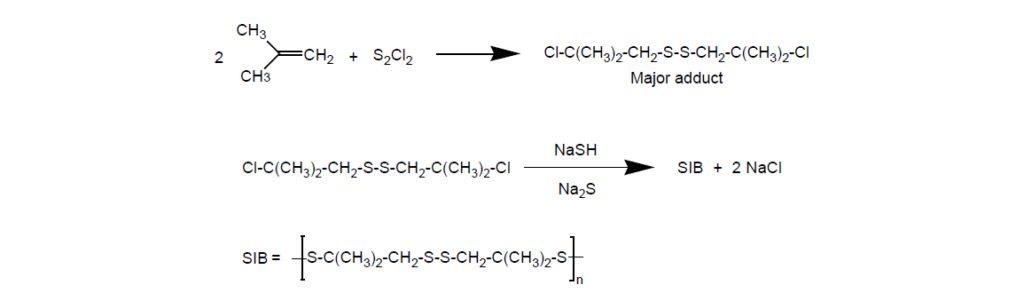
Sulfurized olefins function mainly through thermal decomposition mechanisms. Sulfur prevents contact between interacting ferrous metal surfaces through the formation of an intermediate film of iron sulfide. By doing this, sulfur usually decreases the wear rate but accelerates the smoothing of the surfaces. This smoothing actually helps reduce the wear rate. Furthermore, a higher percent of active sulfur in a molecule increases the chances of reaction with the metal surface and favors EP (antiseizure) more than antiwear properties.
| Surface | Friction Coefficient |
| Steel : Steel | 0.78 |
| FeS : FeS | 0.39 |
| Copper : Copper | 1.21 |
| CuS : CuS | 0.74 |
| Material | Dimension (Å) |
| Size of oil molecules | 50 |
| Size of sulfide layers | 3000 |
| Superfinish surfaces | 1000 |
II. Sulfurized Esters:
Sulfurized esters can be made by sulfurization of unsaturated fatty acid esters such as oleic acid methyl ester.
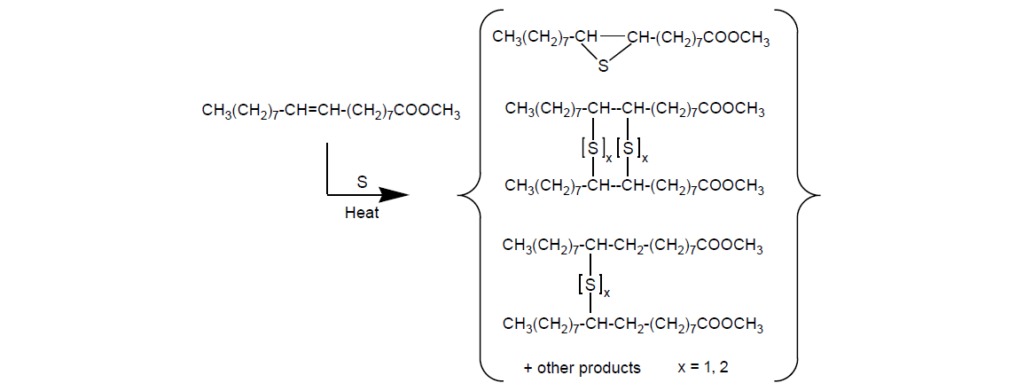
Although the sulfur content may not be as high as in many sulfurized olefins, sulfurized esters are attractive for their exceptionally good frictional properties in many applications. This is because combining sulfur with fat in a lubricant additive provides a synergistic effect [fat provides reduced friction, and sulfur provides wear and EP protection].
Sulfurized esters are polar species that tend to be absorbed in layers of molecular dimensions at the metal interface. The interposition of such films is effective in preventing metal seizure under conditions of EP or under conditions tending to displace the lubricating film between the bearing surfaces. Film strength implies that metal-to-metal contact and welding are prevented as a result of the film formation (or replenishment) by the chemical reaction of the metal and an EP additive.
2. Phosphorus Additives
Phosphorus-containing additives are used to provide protection against moderate to high pressure, metal-to-metal contacts in boundary lubrication and EHL. Unlike sulfur additives, where their EP activity must be balanced against performance requirements for thermal stability and mild corrosivity toward copper-containing alloys, phosphorus additives usually possess very good corrosivity control. Owing to totally different mechanisms involved in surface film formation rates and film strengths, phosphorus additives cannot replace sulfur additives in many applications and vice versa. Typically, phosphorus additives are extremely effective in applications with slow sliding speeds and high surface roughness.
I. Phosphate Esters:
Phosphate esters are produced by reaction of phosphoryl chloride with alcohols or phenols:
3 ROH + POCl3  O=P(OR)3 + HCL
O=P(OR)3 + HCL
Although phosphate esters are widely used as antiwear additives for lubricants, the concerns about hydrolytic stability, thermal stability, and of course, satisfactory antiwear properties are equally important. In that sense, triaryl phosphates are dominant over trialkyl phosphates, because their hydrolytic–thermal stability is much better.
The most commonly used phosphate esters for antiwear performance features are tricresyl phosphates (TCP), trixylenyl phosphates (TXP), and tributylphenyl phosphates (TBP).
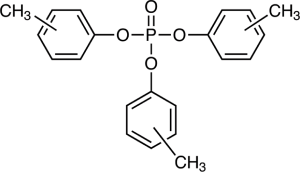
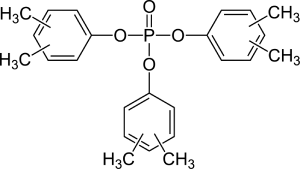
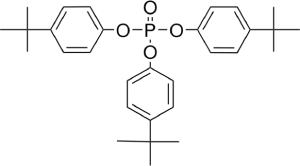
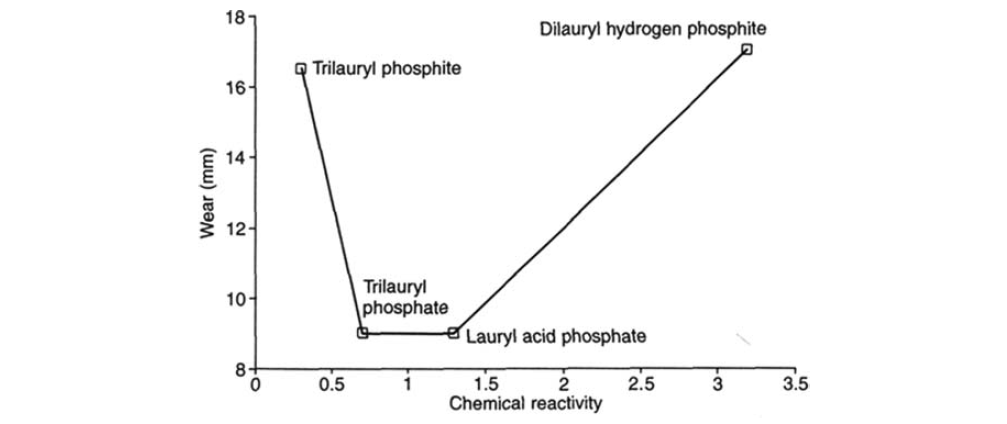
II. Phosphite ُEsters:
In addition to their important role as antioxidants, phosphites are also found to be useful antiwear additives.
Dialkyl hydrogen phosphites and diaryl hydrogen phosphites are neutral esters of phosphorus acid. These materials have two rapid equilibrating forms: keto form and acid form. Physical measurements indicate that they exist substantially in the keto form, associated in dimeric or trimeric groupings by hydrogen bonding.
(RO)2P(=O)H
Keto Form
(RO)2P–O–H
Acid Form
Trialkyl phosphites and triaryl phosphites are neutral trivalent phosphorus esters. These materials are clear, mobile liquids with characteristic odors.

Dialkyl (or diaryl) hydrogen phosphites, besides being excellent antiwear agents, are considered the most potent form of phosphorus, suited to high-torque, low-speed operations. This is the area where antiwear processes are taken to the extreme and is one of the most important sections of the EP performance spectrum. Sulfur can be quite incapable of giving protection under such conditions. Only a phosphorus source, if active enough and in sufficient concentration, can help here. Conversely, phosphorus components are of little use in high-speed and shock operations where sulfur components can be excellent.
With dialkyl phosphites, it has been reported that oxidation produces a phosphate anion, which tends to act as a bridging ligand to form an oligomeric iron (III) complex, that is, an iron oxide complex. However, there is also a weak, high-viscosity, nonsolid film that increases the overall thickness of the total film at high speeds.
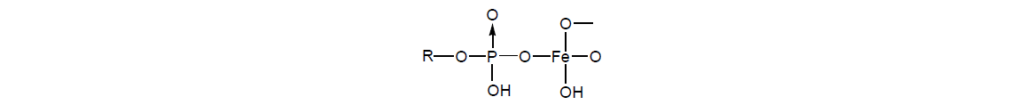
Dialkyl phosphites are widely used in gear oils, automatic transmission fluids (ATF), and many other applications. Spiro bicyclodiphosphites are also reported to be used in continuously variable transmission fluids.

3. Sulfur-Phosphorous Additives
It has been known for many years that sulfur compounds form a film of iron sulfi de, and phosphorus compounds form iron phosphate, on the mating metal surfaces. Generally, the films formed from sulfur sources such as SIB are expected to contain FeS, FeSO4, as well as organic fragments from the additive decomposition. With phosphorus sources, such as dialkyl phosphites, films containing FePO4, FePO3, as well as organic fragments are expected. When both sulfur and phosphorus are present, both elements contribute to the nature of the film, and which one predominates depends on the S/P ratio, the decomposition mechanisms, and the operating conditions, for example, high speed and shock or high torque/low speed.
Sulfur–phosphorus additives are used to provide protection against moderate to high pressure, metal-to-metal contacts in boundary lubrication, and EHL. Metallic sulfur–phosphorus additives, such as zinc dithiophosphates (ZnDTPs), are the most important antiwear/EP components used in engine oils. Ashless sulfur–phosphorus additives are used less extensively, and the most commonly available S/P additives in the marketplace are based on chemistries of dithiophosphates, thiophosphates.
I. Zinc dialkyldithiophosphates – ZDDPs
ZDDP is an organometallic compound having four sulfur atoms coordinated to the zinc atom, which is in a tetrahedral, sp3 hybridized state. A Raman spectrum of ZDDP shows a strong P–S symmetric stretching band near 540 cm–1 and the absence of a strong Raman band near 660 cm–1, indicating a symmetrical sulfur–zinc coordination arrangement as in the following structure:

The neutral ZDDP exist as monomer, dimer, trimer, or oligomer depending on the state of the ZDDP, crystalline or liquid, the concentration of ZDDP in solvent, and the presence of additional compounds.
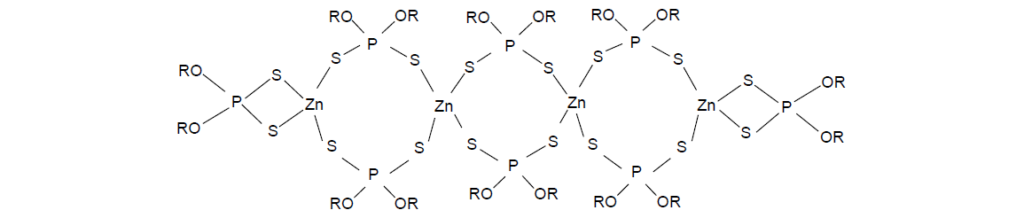
ZDDPs operate mainly as antiwear agents but exhibit mild EP characteristics. As an antiwear agent, ZDDP operates under mixed lubrication conditions with a thin oil film separating the metal parts. Surface asperities, however, intermittently penetrate the liquid film, giving rise to metal-on-metal contact. The ZDDP reacts with these asperities to reduce the contact. Likewise, when the load is high enough to collapse the oil film, the ZDDP reacts with the entire metal surface to prevent welding and to reduce wear. A great deal of study has been done to determine the nature of this protective film and the mechanism of deposition, where the thermal degradation products of the ZDDP are the active antiwear agents.
The antiwear film thickness and composition are directly related to temperature and the extent of surface rubbing:
Initially, ZDDP is reversibly absorbed onto the metal surface at low temperatures.
As the temperature increases, catalytic decomposition of ZDDP to dialkyldithiophosphoryl disulfide occurs, with the disulfide absorbed onto the metal surface. From here, the thermal degradation products are formed with increasing temperature and pressure until a film is formed on the surface.
The antiwear/EP ZDDP film composed of various layers of ZDDP degradation products. Some of these degradation products are reacted with the metal making up the lubricated surface. The composition of the layers is temperature-dependent.
II. Ashless Dithiophosphates
Unlike ZnDTP, ashless dithiophosphates are usually not as versatile, and therefore cannot be considered as multifunctional additives. Although ashless dithiophosphates have fairly good antiwear and EP properties, their anticorrosion properties are not as good as ZnDTP. This is closely related to the stability and decomposition mechanisms of ashless dithiophosphates. Relatively weak corrosion protection also limits their application at high concentrations in engine oils as well as some industrial oils.
Ashless dithiophosphates can be useful in metalworking fluids, automotive transmission fluids (ATF), gear oils, greases, and non-zinc hydraulic fluids.
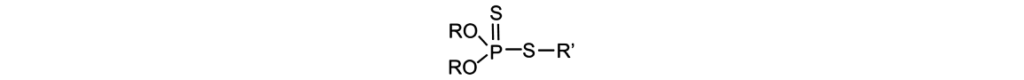
The most common ashless dithiophosphate used in the marketplace is a dithiophosphate ester made from ethyl acrylate and o,o-diisopropyl dithiophosphoric acid as described in the following:
[C3H7–O–]2–P(=S)S–CH2–CH2–C(=O)O–C2H5
III. Ashless Thiophosphate
Ashless thiophosphates are widely used as replacements for metallic dithiophosphates in many lubricant applications where metal is less desirable. Thiophosphates are often present (generated in situ) in lubricant formulations when both sulfur and phosphorus additives are used. Aryl phosphorothioates provide good thermal stability and good antiwear/EP properties as evidenced by their strong FZG performance.

4. Sulfur-Nitrogen Additives
Sulfur and nitrogen-containing additives are used to provide protection against moderate to high pressure, metal-to-metal contacts in boundary lubrication, and EHL. Both open chain and heterocyclic compounds have attracted a considerable amount of research effort to explore their potential as antiwear and EP additives.
I. Dithiocarbamates
Molybdenum dialkyl dithiocarbamate (MoDTC) is a friction modifier lubricant additive widely used alone or in combination with anti-wear additives such as zinc dialkyldithiophosphates (ZDDP).
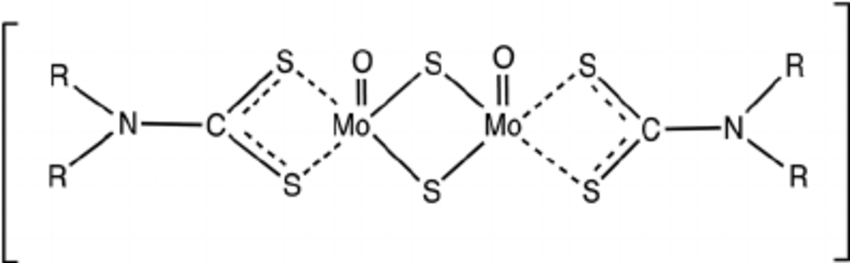
The friction reduction properties of MoDTC are known to be a consequence of the formation of molybdenum disulphide (MoS2) flakes at the asperity peaks of rubbing surfaces, with a size of about 10–30 nm in diameter and a thickness of just a few atomic layers.
II. Dimercaptothiadiazole and Mercaptobenzothiazole Additives:
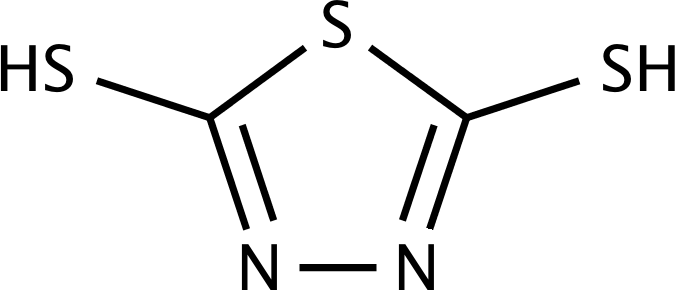
[DMTD]
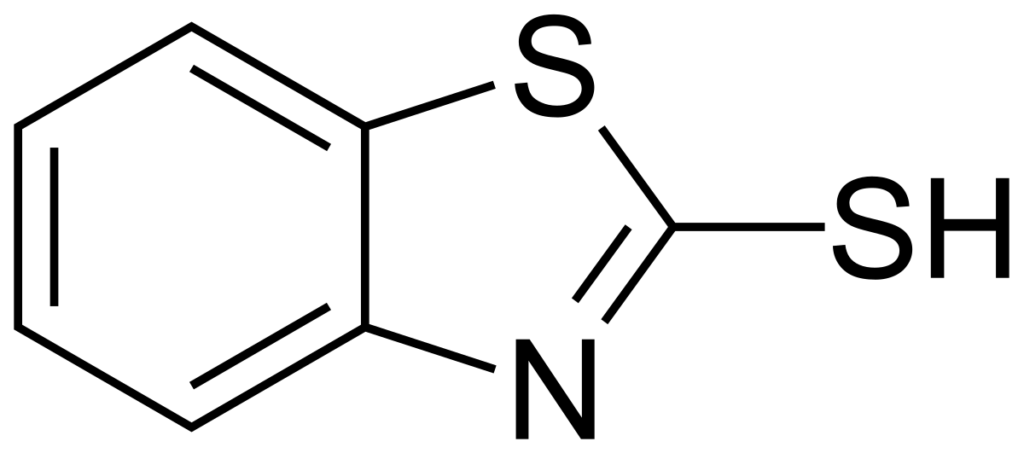
[MBT]
Both MBT and DMTD derivatives are widely used as copper passivators and nonferrous metal corrosion inhibitors. Some proprietary load-carrying additives are substituted MBT and DMTD compounds that are used in various applications either as a component or as a part of additive packages with a specific purpose. In the absence of any phosphorus moiety in MBT and DMTD, their oil-soluble derivatives are suitable for replacing zinc dithiophosphates in some lubricant applications. For example, a commercial, high-density, powder-like MBT and DMTD derivatives is used as a dual functional antioxidant/EP agent in greases.
5. Phosphorous-Nitrogen Additives
Phosphorus–nitrogen additives are used to provide protection against moderate to high pressure, metal-to-metal contacts in boundary lubrication, and EHL. Phosphorus–nitrogen additives are used as dual functional antiwear/antirust additives extensively.
I. Amine Phosphates:
Amine phosphates are extensively used in industrial oils, greases, and automotive gear oils. They offer very good rust protection and also show very good antiwear/EP. Since amine phosphates are very polar species, they interact strongly with other additive components, making their performance very dependent on the formulation. Hence, extra attention is needed when amine phosphates are used.
I. Amine Thiophosphates and Dithiophosphates:
Amine thiophosphates and dithiophosphates are also multifunctional additives providing good rust inhibition and antiwear properties. Owing to their high activity and low stability, amine thiophosphates and dithiophosphates are not as extensively used as either amine phosphates or metallic dithiophosphates.
6. Halogen Additives
Chlorine was one of the earliest antiwear and EP elements used in the lubricant industry. Chlorine-containing additives are still used in cutting oils and related metalworking lubricants, in combination with sulfur additives.
Iodine was mentioned in aluminum-processing lubricants for wear control.
Fluorine, in perfluorinated compounds, is well known to reduce wear and especially friction.
The chlorine compounds act and function in that they coat the metal surface with a metal chloride film under the influence of high pressure at point of lubrication and in the presence of traces of moisture. FeCl2 melts at 672°C and has low shear strength when compare with steel.
The effect of chlorine compounds depends on the reactivity of the chlorine atom, temperature, and concentration. Hydrogen chloride formed in the presence of larger quantities of moisture can cause severe corrosion of the metal surfaces. As the corrosion hazards increase along with the EP properties with increasing reactivity of the chlorine atoms, a compromise must be found in the development of chlorine-containing additives.
Chlorinated paraffins such as trichlorocetane represent a group of important EP additives used in the past. They can significantly increase the load stages in the FZG test with increasing concentration. The chain length has practically very little influence on the EP effect; on the contrary, the load-carrying capacity increases with increasing degree of chlorination. In practice, chlorinated paraffins with ~40 to 70 wt% chlorine are used; however, they are sensitive to moisture and light and can easily evolve hydrogen chloride. Compounds such as phenoxy-propylene oxide, amines, or basic sulfonates neutralize hydrogen chloride and thus act as stabilizers.
Good results are also obtained with chlorinated fatty acids and their derivatives; particularly those with trichloromethyl groups in the end position, since the additives with CCl3 groups are particularly effective.
Owing to their high stability, chlorinated aromatics have less favorable EP properties than the chlorinated aliphatics. Alkylaromatics with chlorinated side chains improve the load-carrying capacity much more than those chlorinated in the ring; the efficiency increases with the number of carbon atoms in the side chain. Chlorinated fatty oils and esters as well as chlorinated terpenes and amines have also been patented as EP additives.
Sulfur–chlorine additives were found to be satisfactory for gear lubrication in passenger cars in the mid-1930s. Apparently, this type of additive could satisfy the high-speed and moderate-load operation of passenger cars used in that time period. When sulfur and chlorine are combined in the organic molecule, sulfur somewhat reduces the corrosive tendency of chlorine; on the contrary, the EP properties of the combined moieties are improved in comparison with the individual compounds. Chlorinated alkyl sulfides, sulfurized chloronaphthalenes, chlorinated alkyl thiocarbonates, bis-(pchlorobenzyl) disulfide, tetrachlorodiphenyl sulfide, and trichloroacrolein mercaptals [Cl2C=CCl–CH(SR′)–SR″, where R′ and R″ are alkyl or aryl] must be mentioned in this class. Reaction products of olefins and unsaturated fatty acid esters with sulfur chlorides contain highly reactive β-chlorosulfides, which due to their reactive chlorine and sulfur atoms give very good EP agents, yet show more or less strong corrosive tendencies. However, severe wear was frequently encountered in truck axles where performance under high-torque, low-speed conditions is of greater importance. Later on, the presence of chlorine, although a good EP agent, was found to be detrimental to lubricant thermal stability. Hence, for the past 30 years, chlorine has not been used in gear oils.
Chlorinated trioleyl phosphate, condensation products of chlorinated fatty oils with alkali salts of dithiophosphoric acid diesters, and reaction products of glycols with PCl3 are examples of chlorine–phosphorus additives used in earlier years.
The most serious drawback for chlorine antiwear and EP additives is in the environmental area. Legislation around the industrial world limits the chlorine content of many lubricants to parts per million. Therefore, except for the cutting oil industry, which is also under pressure to change, chlorine additives are not considered a viable option for modern lubricants.
Evaluation of Antinwear and Extreme Pressure Additives
Specifications for antiwear/EP additives focus primarily on application, base oil compatibility, and quantification of elemental constituents. In addition, specifications typically identify specific and critical performance standards for applications.
| Chemical Class | Property | Performance Test |
| Amine phosphates | Percent of nitrogen, phosphorus, and TAN/TBN | Four-Ball (wear, EP), FZG, rust/oxidation test |
| Methylene bis-dialkyl dithiocarbamate | Percent of sulfur, nitrogen, and residual chlorine, amine | Four-Ball Wear, Four-Ball EP, rust/oxidation test |
| Sulfurized lard, esters, fatty acids | Percent of total sulfur and active total sulfur | Four-Ball (wear, EP), stick-slip, Cu corrosion |
| Triphenyl phosphorothioate | Percent of sulfur, phosphorus, and melting point | Four-Ball EP, FZG, Falex EP, rust/oxidation test |
| Chlorinated paraffins, fatty acids | Percent of chlorine and acid value | Four-Ball Wear, Falex EP, Timken, Cu corrosion |
1. Four-Ball Wear and EP Test:
This tester was developed to evaluate the antiwear, EP, and antiweld properties of lubricants under conditions of high unit pressures and various sliding velocities. Two of the standard tests run on the Four-Ball machine are Mean-Hertz Load and Load-Wear Index.
The Four-Ball Wear tester consists of four 1.5 in. diameter steel balls arranged in the form of an equilateral tetrahedron. The three lower balls are held immovably in a clamping pot, while the fourth ball is made to rotate against them. Test lubricant is added in the test pot, covering the contact area of the test balls. During a test, wear scars are formed on the surfaces of the three stationary balls. The diameter of the scars depends on the load, speed, temperature, duration of run, and type of lubricant.
The Four-Ball EP tester runs at a fixed speed of 1770 ± 60 rpm and has no provision for lubricant temperature control. A microscope is used to measure the wear scars. The procedures involve the running of a series of 10 s tests over a range of increasing loads until welding occurs.
From the scar measurements, the mean load (loadwear index) is calculated and it serves as an indicator of the load-carrying properties of the oil being tested.
2. FZG Four-Square Gear Test Rig:
The FZG test equipment consists of two gear sets, arranged in a four-square configuration, driven by an electric motor. The test gear set is run in the test fluid, while increasing load stages (from 1 to 13) until failure. Each load stage is run for a 15 min period at a fixed speed. Two methods are used for determining the damage load stage.
The visual rating method defines the damage load stage as the stage at which more than 20% of the load-carrying flank area of the pinion is damaged by
scratches or scuffing.
The weight loss method defines the damage load stage as the stage at which the combined weight loss of the drive wheel and pinion exceeds the average of the weight changes in the previous load stages by more than 10 mg.
The test is used in developing industrial gear lubricants, ATFs, and hydraulic fluids to meet various manufacturers’ specifications.
3.Falex EP/Wear Tester:
The Falex test machine provides a rapid method of measuring the load-carrying capacity and the wear properties of lubricants. The test consists of rotating a test pin between two loaded journals (V-blocks) immersed in the lubricant sample. There are two common tests run in this machine:
EP test (subjecting a test lubricant to increasing loads until a failure occurs).
Wear test (subjecting a lubricant to a constant load for a definite period of time while measuring the wear pattern).
4. Timken EP Test:
This test provides a rapid method of measuring abrasion resistance and the load-carrying capacity of lubricants. A number of lubricant specifications require Timken “OK” loads above certain minimum values. The mode of operation consists of rotating a Timken tapered roller bearing cup against a stationary, hardened steel block. Fixed weights force the block into contact with the rotating cup through a lever system. The OK load is the highest load the cup and block can carry without scoring during a 10 min run. Timken abrasion tests are run under fixed loads for extended time periods, and the weight loss of the cup and block are a measure of the abrasion resistance of the lubricant.
5. L-37 High Torque Test:
The CRC L-37 test operates under low-speed, high-torque conditions. It evaluates the load-carrying ability, wear stability, and corrosion characteristics of gear lubricants. The test differential is a Dana Model unit driven by a Chevrolet truck engine and four-speed transmission. A complete, new axle assembly is used for each test after a careful examination of gear tooth and bearing tolerance. After break-in at reduced load and high speed, the test continues for 24 h under low-speed (80 axle rpm) and high torque conditions.
6. L-42 High Speed Shock Test:
The CRC L-42 test is established to evaluate the antiscore performance of EP additives in gear lubricants under high-speed, shock load conditions. The test axle is a Dana Model unit driven by a Chevrolet engine through a four-speed truck transmission. The procedure requires fi ve accelerations in fourth gear with inertia loading and 10 accelerations in third gear with dynamometer loading. The lubricant evaluation is based on the amount of scoring, and test results are expressed as percent tooth contact area scored.
7. FAG FE-8 Test:
FAG developed this test frame to be a flexible tribological system to conduct tests over a wide range of operating conditions with different test bearings. Short duration
standardized tests have been developed for different applications. FAG also uses longer-term testing (e.g., fatigue) for comprehensive evaluations. The FE-8 gear oil test was developed specifically to evaluate the effectiveness of antiwear additives. The test runs under heavy load and low speed that forces the bearing to operate under boundary lubrication conditions.
Other tests including Optimol SRV, Cameron-Plint, high-frequency reciprocating rig (HFRR), Falex multi-specimen, Vickers vane pump, Vickers 35-VQ-25 pump, and Denison high- pressure pump tests are also used widely in evaluation of various lubricants and greases. Appropriate fi eld tests are also arranged in proprietary test sites to ensure good product quality and equipment compatibility/friendliness before the introduction of a new product into the marketplace.
Evaluation of Antinwear and Extreme Pressure Additives For Engine Oils
Passenger Car Oils:
Among the GF-4 tests, the most critical engine tests related to antiwear/EP performance are the Sequence IVA and the Sequence IIIG
The Sequence IVA which designed to evaluate an oil’s ability to prevent cam lobe wear in slider valve train design engines operated at low temperature, short trip, and “stop and go” conditions (low-speed/low-temperature operation). Following is a list of the test conditions and specifications:
| Engine | Nissan 2.4 L inline 4 cylinder |
| Engine speed | 800 and 1500 rpm cycles |
| Engine torque | 25 N m |
| Oil temperature | 50 – 60°C |
| Cycle duration | 50 min low speed/10 min high speed |
| Test length | 100 h |
| 7-Point cam lobe wear | 120 μm maximum |
The Sequence IIIG procedure is designed to evaluate the oil resistance to oxidation and wear in high-speed and high-temperature vehicle operation. The test conditions and specifi cations are summarized as follows:
| Engine | GM 3800 Series II V-6 (231 CID) |
| Engine speed | 3600 rpm |
| Engine load | 250 N m |
| Valve spring load | 93 Kg |
| Oil temperature | 150°C |
| Coolant temperature | 115°C |
| Test length | 100 h |
| Average cam and lifter wear | 60 μm maximum |
Heavy-duty Diesel Engine Oils:
The API CJ-4 category requires three tests that include valve train wear as a pass/fail parameter. The Roller Follower Wear Test (ASTM D5966) is run in a 6.5 liter V-8 GM diesel engine; it was initially developed for the older API CG-4 category, which was developed for the introduction of low sulfur (500 ppm maximum) fuel. However, this test has remained as a requirement in all subsequent specifications. At the end of this 50 h test, the used oil soot level is typically 3.5 to 4.0%. The level of wear on the stationary pin in the hydraulic cam followers is measured. The Cummins ISB test (ASTM procedure in progress) was introduced as an industry requirement for API CJ-4.
This 350 h test runs in a 5.9 liter in-line 6 cylinder engine running of ultra low sulfur (15 ppm maximum) diesel fuel. The first 100 h are run at steady-state conditions to generate 3.25% soot in the oil. The final 250 h are run under cyclic conditions to stress cam and tappet wear, which are the primary pass/fail criteria. The third diesel engine test that measure valve train wear is the Cummins ISM test (ASTM procedure in progress). The Cummins ISM is the third in a series of Cummins heavy-duty wear tests developed for API and engine builder diesel oil specifications. Similar to previous Cummins M11 HST (ASTM D6838) and Cummins M11 EGR (ASTM D6975) tests, the Cummins ISM alternates between 50 h soot generation and 50 h wear stages. This test runs for 200 h using 500 ppm sulfur diesel fuel. The used oil typically contains 6 to 7% soot, and the key pass/fail wear parameters are focused on the crossheads (bridges for the inlet and exhaust valves) and the adjusting
screw for the fuel injectors.
The Mack T-12 test (ASTM D7422) measures ring and liner wear under severe operation using 15 ppm sulfur fuel. This 300 h test runs with a very high EGR rate for the first 100 h to generate 4.3% soot. During the final 200 h, the engine runs over-fueled at peak torque conditions to create a very severe environment for top ring weight loss and liner wear at the point of top ring reversal, which are the key wear parameters for this test.

Facebook Comments