Endless Improvements
العربية
Pour Point Depressant
Pour point depressants (PPDs), also known as low-temperature flow improvers and wax crystal modifiers, are polymeric molecules that are added to mineral oil-based lubricants to improve their cold flow properties. Without the addition of a PPD, many lubricants at cold temperatures would be too viscous to flow easily, or might even be gelled, and the result would be little or no lubricant moving through the system or machine requiring lubrication.
For various lubricant applications such as automatic transmission fluids (ATFs), engine oils, gear oils, and hydraulic fluids, paraffinic base stocks are the preferred lubricant. These paraffinic base stocks are typically derived from crude petroleum and are composed of nonaromatic saturated hydrocarbons. Paraffinic stocks make excellent lubricants because they are chemically stable, resistant to oxidation, and have good viscosity index values. However, paraffinic stocks, by their very nature, contain molecular species that have linear carbon chains of 14 carbons or more. These species, recognized as waxy materials, can cause oil pumpability failures at low temperatures. In addition to the inherent waxy base stock components, other sources of waxy material are added to the lubricant as part of its product-specific formulation. These additional components include some viscosity index improvers (VII) and components of the detergent-inhibitor (DI) package.
Fluid Mechanism and Pour Point Depressant Mechanism of Action
Mineral oils are commonly understood to be Newtonian fluids, meaning that they behave according to the following equation: Shear stress = shear rate × viscosity
Experiments show that this equation holds for mineral oils as long as the temperature is above the cloud point of that oil. The cloud point is the temperature at which some of the waxy components of a mineral oil start to crystallize and precipitate from solution, leading to a hazy appearance. A plot of log–log viscosity versus log temperature is:
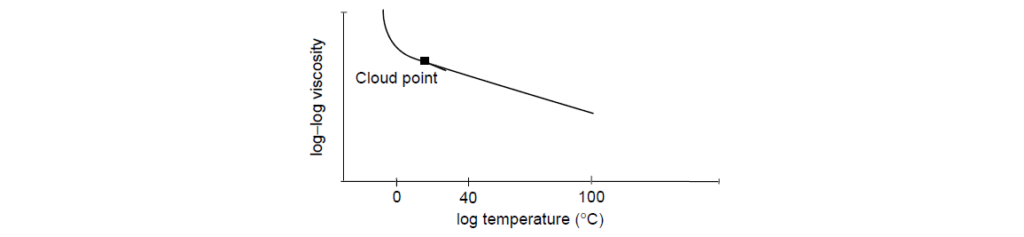
Above the cloud point the viscosity decreases proportionally with temperature.
At temperatures below the cloud point, the viscosity increases steeply as the temperature decreases.
Below the cloud point, it is not uncommon to observe one of the two non-Newtonian behaviors in these otherwise Newtonian fluids:
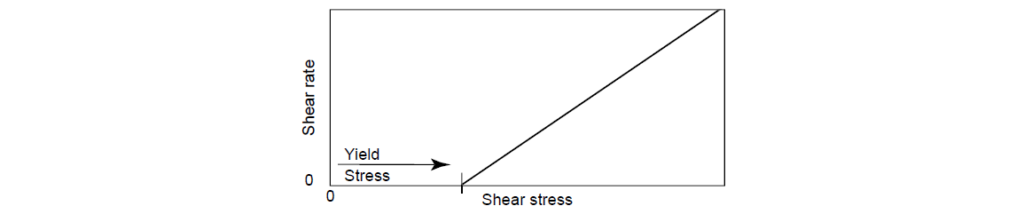
1. Bingham Fluid Behavior:
Bingham fluid behavior is the failure of a fluid to move under low-shear conditions, that is, unless some energy is added to the system (behaves as a rigid body at low stresses but flows as a viscous fluid at high stress).
The equation describes Bingham fluid behavior: Shear stress = (shear rate – yield stress) × viscosity
This failure to flow is similar to the initial flow observed on opening a bottle of ketchup. When a bottle of ketchup is turned over, the ketchup does not flow out of the bottle due to weak associations among some molecular components of the ketchup. However, adding energy to the system by tapping the bottom of the bottle triggers the ketchup to move. Likewise, mineral oils at cold temperatures often do not flow because they contain molecules that have a crystalline nature at low temperatures.

First, two-dimensional crystals (platelets) form and then ultimately three-dimensional needle-like structures form. The needlelike structures layer on one another, forming a network of crystals that trap the non-crystalline oil molecules within the gel network, which impedes the flow of the oil. This process is known as gelation, and the source of the yield stress (YS) in the preceding equation is the wax–gel matrix in which the non-crystalline oil molecules are immobilized.
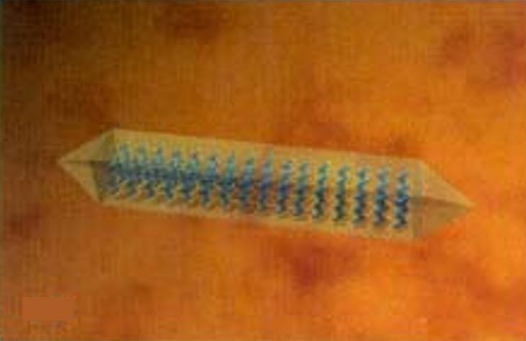

Bingham fluid behavior can be observed by running two widely recognized industry tests:
ASTM D 4684: Standard Test Method for Determination of Yield Stress and Apparent Viscosity of Engine Oils at Low Temperature.
ASTM D 5133: Standard Test Method for Low Temperature, Low Shear Rate, Viscosity/Temperature Dependence of Lubricating Oils Using a Temperature-Scanning Technique [a high gelation index (>12) measurement].
Air binding is an example of problematic Bingham fluid behavior that can occur in an automobile engine. When the engine is at rest, the oil drains and collects in the oil pan. At cold temperatures, the waxy materials crystallize in the oil creating a gel network. When the engine is started, there is enough energy created by the oil pump to break apart the fragile gel structure just at the oil filter. A small bit of oil is pumped into the engine leaving an air gap at the filter. It is possible that the oil in the pan is of a viscosity that it could pump, but is locked in place by the wax crystal network that has formed. At this point, air is being pumped into the engine and as air is a very poor lubricant, this can lead to engine failure.
2. High-Viscosity Behavior:
The second non-Newtonian behavior often observed in a mineral oil at temperatures below its cloud point is unpredictably high viscosity. As waxy molecules in the oil crystallize, they co-crystallize but possibly do not form a strongly organized network. However, these co-crystals have enough hydrodynamic volume that they impede the flow of the non-crystalline oil molecules, greatly increasing the viscosity of the oil. In this situation, the oil is so viscous that it cannot be pumped at all into the engine, creating a serious situation where the engine runs with limited, if any, lubrication.
It is important to note that flow-limited behavior can occur in an oil for another reason, unrelated to wax crystal precipitation and growth. All fluids will become more viscous as the temperature decreases, eventually reaching a viscosity where the fluid cannot be pumped. The temperature at which the fluid no longer flows under gravity, not due to wax crystal growth, but simply due to the viscosity contribution of the other molecular species present, is called the viscous pour point. The addition of a PPD to a fluid will not change its viscous pour point.
Pour Point Depressant Mechanism of Action:
A Pour Point Depressant can resolve both gel structure and high-viscosity modes of failure. Pour Point Depressants work by controlling the wax crystallization phenomenon in two principal ways:
Delaying the formation of wax–gel matrix to significantly lower temperatures than would normally occur or
Reducing the viscosity contribution of the crystal wax particles.
Pour Point Depressant Chemistry
Before the use of Pour Point Depressants, options were few for controlling wax crystallization.
One method was to use heat. For example, fires were built under the oil reservoir of a vehicle.
Another technique was to increase the solvency of the lubricant fluid portion by the addition of kerosene. The kerosene would then evaporate during use.
A third alternative was the use of naturally occurring materials such as microcrystalline waxes or asphalt resin.
The chemical structure of a Pour Point Depressant resembles a comb polymer; long waxy side chains are added into the polymer backbone and interspaced with short neutral (non-wax interacting) side chains.
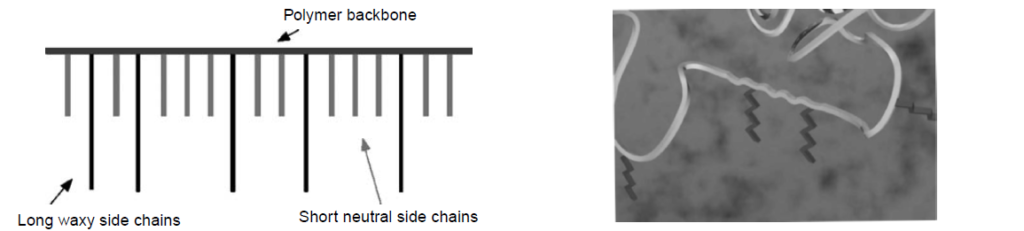
The backbone molecular weight of a Pour Point Depressant, generally, can vary with little effect on performance; however, there is a minimum backbone size below which a Pour Point Depressant will become largely ineffective. Also, a distribution of hydrocarbon side chain lengths can be utilized for best interaction with the wax in the oil because the wax contains a range of molecule chain lengths.
The long waxy side chains can be linear or branched and should contain at least 14 carbon atoms for the Pour Point Depressant to interact with the wax in the oil. These side chain crystallizing with the oil crystals on the edges of the platelets. This co-crystallization sterically inhibits the formation of the three-dimensional network, thus preserving the wax as a distribution of tiny crystals and ensuring the complete fluidity of the oil.
The short neutral side chains act as inert diluents and help to control the extent of wax interaction.
The intensity of interaction between the wax and the alkyl side chains is a function of the types and amounts of side chains. This interaction can be expressed by a wax interaction factor (WIF). WIF is a method of ranking Pour Point Depressants by their wax nature, accounting for the amount of waxy side chains in a Pour Point Depressant and their strength of interaction. A low-wax fluid usually responds best to a low-WIF Pour Point Depressant, and likewise, optimum performance per unit will be achieved in a more waxy fluid with a higher-WIF Pour Point Depressant. Because of the need to treat a vast variety of wax-related situations, a multitude of Pour Point Depressant products have been developed.
As described, the mode of operation for a Pour Point Depressant is physical interference. In selecting an optimum Pour Point Depressant, consideration needs to be given to the nature and amount of waxy molecules that may be present in a fluid. The waxy side chains present in the Pour Point Depressant need to be in balance with those waxy molecules found in the fluid. This balance is critical to blending oils with good low-temperature flow properties.
Pour Point Depressant Performance Testing
Mineral oil is typically non-Newtonian at low temperatures; therefore, no single test can ensure that a lubricant blended with mineral oil will remain fluid over a wide range of conditions. Thermal history, including temperature cycling and cooling rates, can also affect the flow behavior of the oil. Various test methods have been developed over the years to evaluate oils under conditions that are experienced during operation.
A test, to be meaningful in predicting wax crystal growth, must incorporate three critical factors: a low-temperature end point, a defined cooling profile, and a low shear rate.
Obviously, wax crystal growth is a low-temperature phenomenon, so a low-temperature end point is required. The low temperature finally achieved in a test needs to reflect the temperature requirements of the application. Not all waxy molecules in a fluid are crystalline at the same temperature, and changing the temperature point for a measurement will greatly impact the quantity of wax present as a crystal; hence, making an appropriate choice of a final test temperature is critical. The rate of cooling affects the competing factors of crystal growth and nucleation of new crystals, thus affecting the number of crystals formed and their relative sizes. The existence of temperature cycling within the cooling profile can further affect the number or size of wax crystals. Clearly, a defined cooling rate is critical in understanding wax crystal growth. Lastly, low shear rate is also a critical part of characterizing low-temperature behavior. Wax networks are fragile and easily disrupted under higher shear conditions. To understand whether wax crystal networks have formed, it is important to study the fluid under low shear conditions, allowing any network that formed to be preserved.
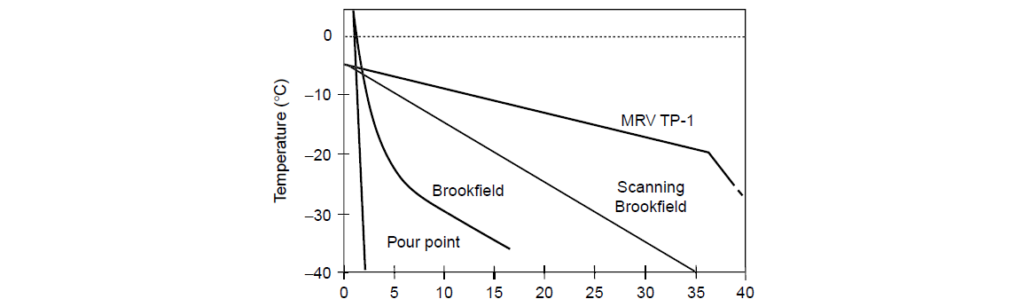
| Test | Cooling Rate (ºC) | Shear Rate (s-1) | Test Duration |
| ASTM D 97(a) | 0.6/min | 0.1–0.2 | 2 h |
| Stable Pour Point | 0 to -40 over 7 days | 0.1–0.2 | 7 days |
| ASTM D 3829(b) | 2/h | 17.5 | 16 h |
| ASTM D 4684(c) | 0.33/h | 17.5 | 48 h |
| ASTM D 2983(d) | Shock | 0.1–0.12 | 16 h |
| ASTM D 5133(e) | 1/h | 0.25 | 35 h |
| (a) Pour Point. | (c) Mini-Rotary Viscometer Temp. profile 1. |
| (b) Mini-Rotary Viscometer. | (d) Brookfield Viscosity. |
| (e) Scanning Brookfield Viscosity and Gelation Index. | |
The ASTM D 97 test for pour point employs a rapid and unrealistic cooling rate for wax crystal growth. Quick cooling does not allow the wax crystals that form to fully associate. However, this test has been used by the industry for many years, and its quickness certainly has benefit in quality control situations.
The stable pour point is more realistic than the ASTM D 97 pour point because of its longer cooling time.
ASTM D 3829 and ASTM D 4684 have comparable shear rates, but ASTM D 3829 employs a programmed cooling rate over a shorter time period, on average 2°C/h over 16 h, versus 0.33°C/h over ∼48 h for ASTM D 4684.
Because of the differences in the tests, analysis of several test method results is critical to selecting the best Pour Point Depressant for a lubricant formulation. A Pour Point Depressant that gives satisfactory performance at both rapid and slow cooling rates under low shear conditions is presumed to be better able to deliver performance over all foreseeable conditions than a Pour Point Depressant that already shows performance deficits under one of the limiting test conditions. In the end, it is impossible to test all conditions that might possibly arise.
| Percentage PPD | As is | 0.15% | 0.3% |
| Viscosity at -25ºC (mPa s) | 2900 | 2950 | 2850 |
The cold cranking simulator (CCS) test (ASTM D 5293, which replaced ASTM D 2602) is a common oil testing method that is inappropriate for wax gel testing because it uses a rapid cooling rate and high shear rate. One set of results from ASTM D 2602 testing to determine whether Pour Point Depressant addition can affect the CCS viscosity of a Society of Automotive Engineers (SAE) 5W-30 oil. As the Pour Point Depressant content was increased from 0 to 0.3%, virtually no change in the viscosity was observed. This result is contrary to what many hope for with the addition of a Pour Point Depressant and is explained by the high shear nature of the CCS test. Pout Point Depressants are added to a fluid to control wax crystal growth. Wax crystal networks are fragile. Any wax network that formed would be broken up under the high shear conditions of the CCS test and as a result Pour Point Depressants cannot improve the CCS viscosity of an oil.
Principles of Pour Point Depressant Selection
Treat Rate and Pour Point Reversion:
Using a Pour Point Depressant to improve the pour point means the addition of a waxy material to the lubricant. The treat rate, or concentration, of a Pour Point Depressant should be optimized so that the Pour Point Depressant itself does not cause crystallization.
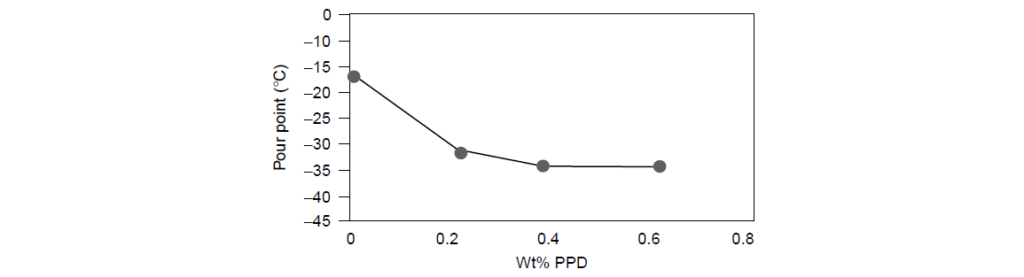
The effect of the Pour Point Depressant treat rate on the pour point of Group I 100N base oil:
Addition of a Pour Point Depressant at 0.2% by weight reduces the pour point from -15 to -33°C.
Doubling the concentration to 0.4% produces only an incremental decrease of ∼3°C.
An additional increase in the Pour Point Depressant content does not reduce the pour point any further.
For this particular oil–Pour Point Depressant system, the optimum treat rate is ∼0.2%.
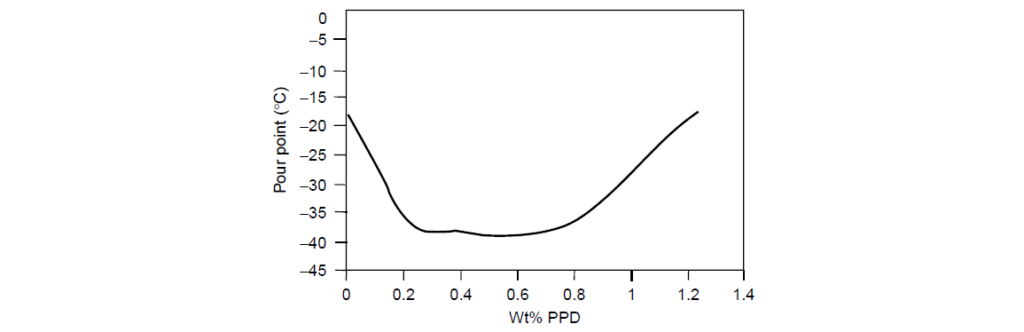
Continued increases in the Pour Point Depressant content result in a phenomenon known as pour point reversion. The addition of Pour Point Depressant beyond the optimum treat rate is in effect adding wax to the system and consequently reversing the benefit of the Pour Point Depressant by contributing to the formation of crystals or Pour Point Depressant polymer networks.
An example of Pour Point Depressant overtreatment of the oil is shown below. At a treat rate of 0.7; the pour point begins to increase at a treat rate of 0.7% from its minimum value at the treat rate of 0.3%. At the highest concentration tested, 1.2%, the pour point was similar to the untreated oil. Pour point reversion can also occur with improper selection of a Pour Point Depressant with a WIF that is too high for the oil being treated.
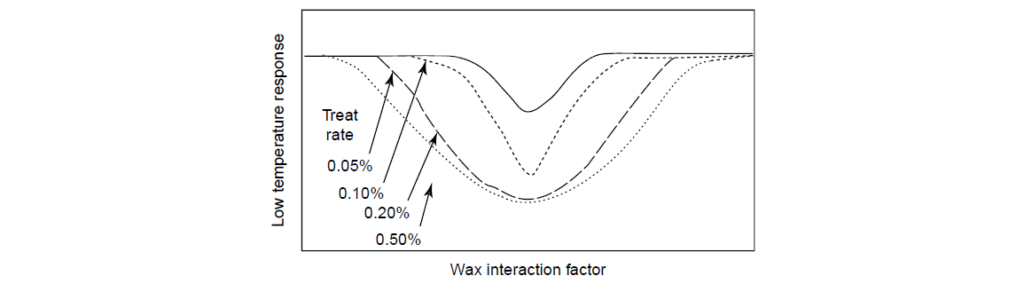
A typical response curve demonstrating the effect of a Pour Point Depressant as a function of the WIF in one specific base oil. The lowest treat rate of 0.05% (top curve) indicates that a specific WIF is responsible for optimum performance. As the treat rate increases, the size of the response window increases. If a less-specific response is desired, then a higher treat rate can be used. Also, WIF and treat rate can, to some degree, be traded off, meaning that a non-optimum WIF can be offset by a higher treat rate and vice versa. This flexibility can allow for one Pour Point Depressant to function well in multiple oils or across plant systems.
Effect of Performance Additives:
I. Base Oil Wax Chemistry and Content
The wax content of base oils from which lubricants are formulated varies with:
The source of the crude oil.
The refining process used for the crude oil.
The dewaxing process used for the refined oil (certainly a key determinant of the low-temperature low-shear behavior of an oil).
The final viscosity grade of the base oil.
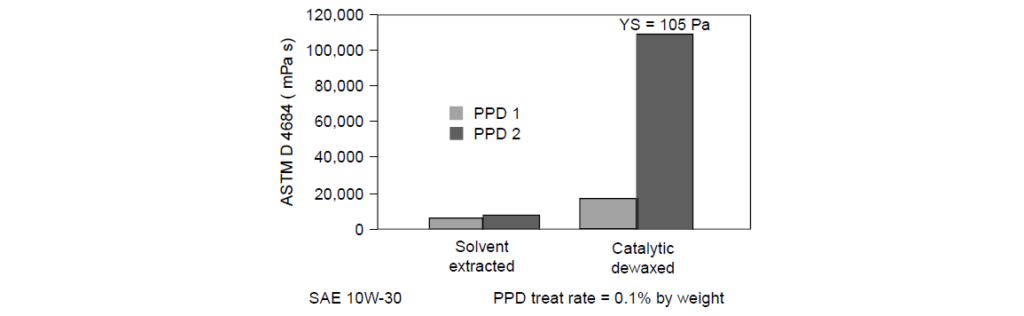
Determining the viscosity of the four Pour Point Depressant–oil formulations:
Two different Pour Point Depressants: PPD1 had a lower WIF than PPD2.
Two different SAE 10W-30 formulated oils: solvent-extracted and catalytically dewaxed (both oils had the same DI and VII).
For the solvent-extracted oil, the viscosity remained fairly low for both Pour Point Depressants. However, the catalytically dewaxed oils had a higher viscosity with PPD2 than with PPD1 and extremely high YS of 105 Pa with PPD2. To pass current North American engine oil specifications, YS must be less than 35 Pa. The higher WIF of PPD2 most likely caused the increase in viscosity and YS in the catalytically dewaxed oil. One explanation for this is that the catalytically dewaxed stock contains fewer linear molecules of C14 or greater. The waxy side chains of the Pour Point Depressant, finding limited natural waxes to interact with, self-associate and built viscosity and structure through polymer-to-polymer interactions.
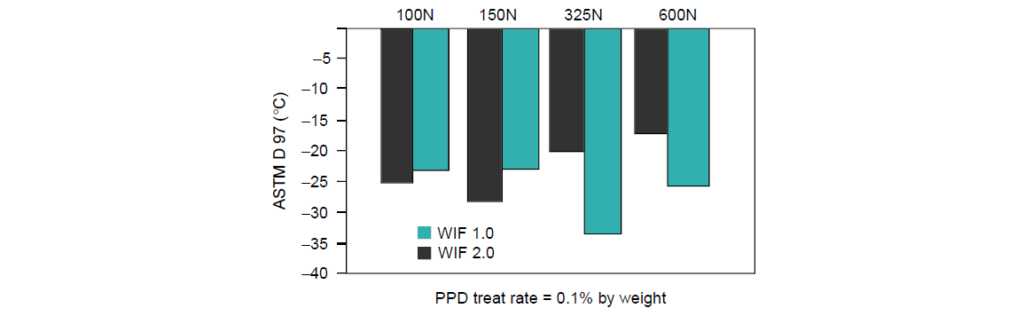
The effect of the WIF of a Pour Point Depressant on different viscosity grades of Group I base oils is:
For the lower viscosity grades of 100N and 150N, increasing the WIF from 1 to 2 had a small effect on decreasing the pour point temperature.
For the higher viscosity grades of 325N and 600N, the result was reversed, and the higher WIF decreases the pour point temperature significantly.
This result can be explained by the interaction of the PPD with the longer-chain hydrocarbons present in the higher viscosity grades.
II. Other Wax Sources:
Other wax sources include DI packages and friction modifiers that may themselves contain waxy hydrocarbon chains. Also, VIIs, which can typically contain long ethylene sequences, should be considered. These waxy components can affect the LTLS performance of the fully formulated oil, both positively and negatively, and therefore it is important to understand their contribution when selecting a PPD for a lubricant.
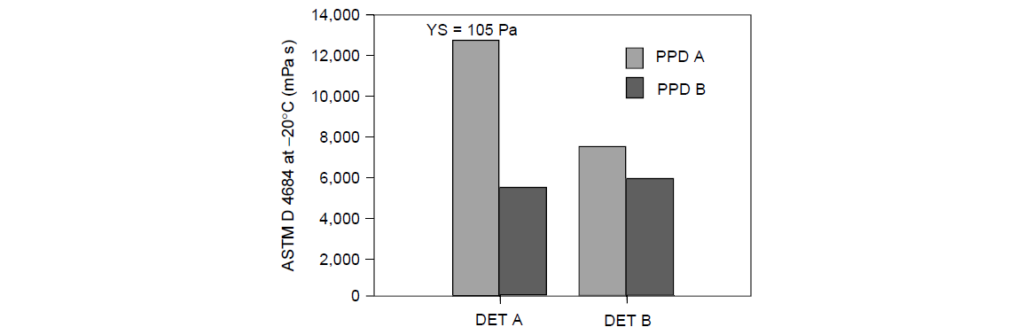
Detergent effects using two different PPDs [PPD A had a lower wax content than PPD B] in SAE 15W-40:
In the oil with detergent A:
PPD A was able to control the viscosity to 13,000 mPa s, which was below the test specification of 30,000 mPa s. However, PPD A could not control the YS, which, at 105 Pa, exceeded the test specification of <35 Pa.
The excessive YS indicated that a gel network was forming. One explanation for this is that detergent A was waxy in nature, and the waxy side chains of PPD A interacted with detergent A leaving fewer Pour Point Depressant side chains available for interaction with the wax in the oil.
PPD B controlled the wax crystallization.
The results for both Pour Point Depressants were comparable in detergent B testing, passing both viscosity and YS specifications.
Chemistry effects of VIIs and a PPD in SAE 5W-30 oil:
At -30°C: oils containing both VII1 and VII2 had comparable performance, and the oils met the test specification of <30,000 mPa s.
As the temperature was lowered to -35°C, a much larger change in viscosity was observed in the oil containing VII2. The oil containing VII1 doubled in viscosity, whereas the oil containing VII2 tripled in viscosity. Although both VIIs met the test specification of <60,000 mPa s at -35°C, this Pour Point Depressant was not able to control viscosity growth in the oil with VII2.
With proper Pour Point Depressant selection, VII2 certainly can show equal performance to VII1. This demonstrates the effect that different cold-temperature conditions can have on Pour Point Depressant selection and how seemingly small changes in an oil formulation, VII1 or VII2, can change the optimum Pour Point Depressant for a lubricant.
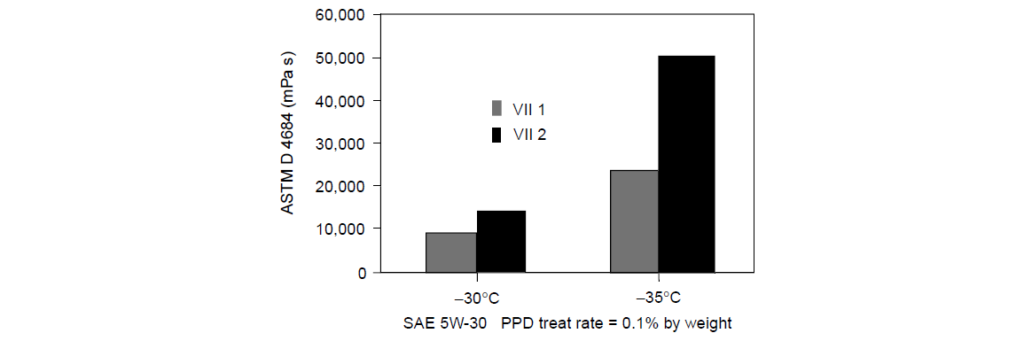
Olefin copolymers (OCPs) are used commonly as VIIs. The ethylene content of the OCP can greatly affect the choice of Pour Point Depressant for optimum viscosity and structure control of a lubricant.
The importance of testing Pour Point Depressants and considering all pertinent tests is summarized as follow:
| VII | Traditional | HE OCP | HE OCP |
| PPD | 1 | 1 | 2 |
| Properties: | |||
| – ASTM D 97 (ºC) | -30 | -27 | -30 |
| – ASTM D 4684, viscosity (mPa s) | 13,700 | 13,900 | 9,700 |
| – ASTM D 5133 (ºC at 30,000 mPa s) | -28.8 | -25.9 | -30.2 |
| – Gelation index | 4.6 | 9.8 | 4.8 |
The data presents the results of testing two different Pour Point Depressants in SAE 10W-30 oil containing either a traditional OCP or a higher ethylene (HE) OCP.
Using PPD1, a lower-WIF PPD, the oil containing the HE OCP had results similar to the oil containing traditional OCP for all the tests except the gelation index, which approached the specification limit of 12, indicating a structure is starting to develop.
When PPD 2, a higher-WIF PPD, was used with the HE OCP, the results were similar to or better than the oil containing traditional OCP and PPD 1. Assuming that the longer ethylene sequences in the HE OCP can contribute to wax interactions, these data demonstrate that a high-WIF PPD is needed to disrupt those waxy VII crystals as well as to interact with the waxy species in the base oil.
III. Fully Formulated Oil:
It should be obvious from the previous discussion that another important aspect of Pour Point Depressant selection is the viscosity behavior of fully formulated oil versus base stocks. The fully formulated oil almost always contains additives and wax sources not present in the base oil, and these waxes may affect the viscosity behavior. The effect of two different PPDs on a 150N base oil and a SAE 10W-40, formulated with that same 150N oil, is:
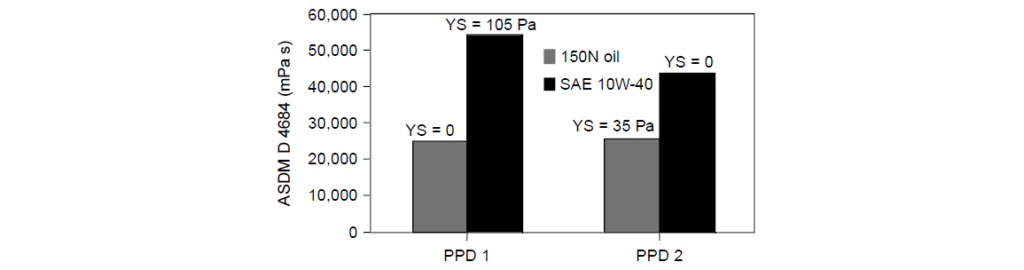
PPD2 has a higher WIF than PPD1.
PPD1 and PPD2 gave similar viscosity results when tested in the base oil alone; however, YS was detected with PPD2. These results indicate that PPD1 would be a better candidate for the 150N base oil.
With the fully formulated oil, however, PPD1 resulted in a higher viscosity and a YS of 105 Pa, which exceeded the test specifications of <35 Pa. In this case, PPD 2 is the better choice because the additional waxy components from the DI or VII in the SAE 10W-40 formulated with that some 150N oil formulation required more waxy sites on the PPD polymer to effectively control the LTLS properties of the fully formulated oil.
Pour Point Depressant Robustness
The usefulness of Pour Point Depressants has been clearly established through laboratory testing. However, the question of whether a Pour Point Depressant loses its effectiveness during use must be considered. The robustness of the Pour Point Depressant can be determined from field and laboratory tests.
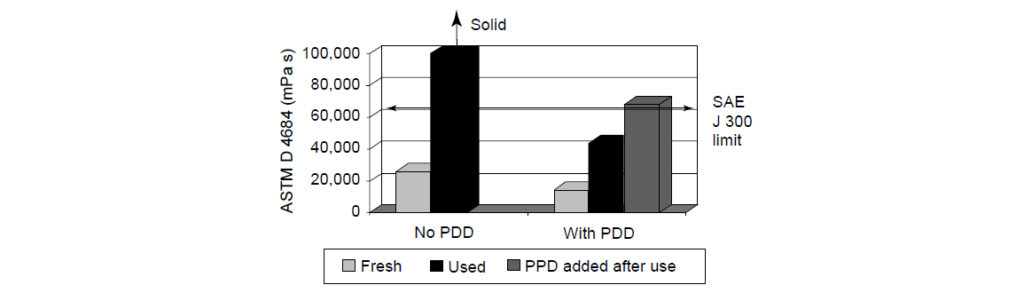
The comparison of the viscosity for:
Fresh oil without Pour Point Depressant at -35°C.
Used oil without Pour Point Depressant at -30°C.
Fresh oil with Pour Point Depressant at -35°C.
Used oil with Pour Point Depressant at -30°C.
Post-test addition of the Pour Point Depressant to used oil at -30°C.
Severe Field Test (New York City 10,000 mi Taxi Test):
Without the Pour Point Depressant, the used oil had a significantly higher viscosity than the fresh oil, becoming too viscous to measure during the testing at -30°C.
On the contrary, the used oil containing Pour Point Depressant easily passed the SAE J 300 limit of 60,000 mPa s with no YS.
Additionally, when Pour Point Depressant was added to the used oil without Pour Point Depressant, the used oil viscosity was greatly improved, showing slightly higher but similar results to the used oil with Pour Point Depressant results.
The Laboratory Test (Sequence IIIG Test – ASTM D 7320):
Same result with filed test.
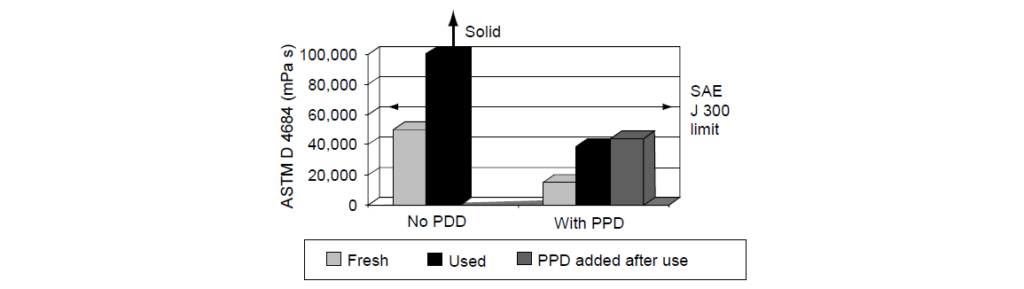
Clearly, the Pour Point Depressant had not deteriorated during use as the PPD-pretreated used oil provides good MRV TP-1 viscosity, the untreated used oil has failing MRV TP-1 viscosity, and the post-added Pour Point Depressant used oil results are similar to the oil pretreated with Pour Point Depressant.
Lubricant Applications
Pour Point Depressants are selected and engineered for different lubricant applications ranging from automotive engine and transmission oils to industrial oils. Engine oils tend to drive the Pour Point Depressant selection process for most blenders as engine oils are required to meet several LTLS specifications with severe limits, and they are often the high-volume products for a blend plant. Additionally, other lubricants more often are less selective for Pour Point Depressant.
Automotive Engine Oil:
A Pour Point Depressant is always needed for automotive engine oils formulated with mineral oil base stocks, and given the complexity of these oils, the optimum Pour Point Depressant can usually only be identified by undertaking a Pour Point Depressant study on the fully formulated oils. The LTLS tests that are most often used today to access the pumpability of engine oils are ASTM D 4684, ASTM D 5133, and ASTM D 97. Various national, international, and OEM-specific engine oil specifications exist, each varying in the low-temperature test recommended or in the acceptable limits for a particular test.
Automotive Transmission Oil:
Selection of Pour Point Depressants for automotive transmission oils, including gear oils and ATFs, normally entails testing with ASTM D 2983 (Brookfield viscosity) at -12, -26, and -40°C. A Pour Point Depressant is always needed for mineral oil–based fluids, but it may be a constituent of the VII, if one is used.
Industrial Oil:
In contrast to the automotive oils, which typically require specialized low-temperature viscosity testing, industrial oils often have just a simple pour point (ASTM D 97) requirement. Therefore, the targets are often not difficult, and a low treat level of a traditional Pour Point Depressant may be suitable. For these oils, base stock choice is the primary driver for Pour Point Depressant selection. Some fluids, such as multi-graded hydraulic or tractor fluids, have additional LTLS requirements, namely Brookfield viscosity. These fluids generally need a low dose of Pour Point Depressant to meet requirements, and a Pour Point Depressant lab study will identify the optimum product and treat rate. Specific industrial oils, such as refrigeration oils, may be blended with synthetic or naphthenic stocks. These oils have excellent LTLS properties, and the addition of a Pour Point Depressant will not generally give further improvement.

Facebook Comments