Endless Improvements
العربية
Additives – Antioxidants
Oxidation produces harmful species, which eventually compromises the designated functionalities of a lubricant, shortens its service life, and to a more extreme extent, damages the machinery it lubricates. The oxidation is initiated upon exposure of hydrocarbons to oxygen and heat and can be greatly accelerated by transitional metals such as copper, iron, nickel, and so on. For the prevention of lubricant oxidation, antioxidants are the key additive that protects the lubricant from oxidative degradation, allowing the fluid to meet the demanding requirements for use in engines and industrial applications.
For more discussion about Lubricant oxidation mechanism click here
The proceeding mechanistic discussion makes clear several possible counter measures to control lubricant oxidation.
Trapping of catalytic impurities by using of a metal deactivator
Since transitional metals are present in most lubrication system, metal deactivators are usually added to lubricants to suppress the catalytic activities of the metals. Based on the functioning mechanisms, metal deactivators for petroleum products can be classified into two major types:
a. Surface Passivators
The surface passivators act by attaching to metal surface to form a protective layer, thereby preventing metal–hydrocarbon interaction. They can also minimize corrosive attack of metal surface by physically restricting access of the corrosive species to the metal surface.
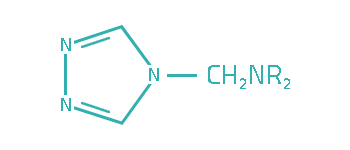
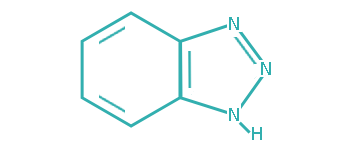
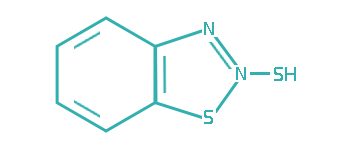
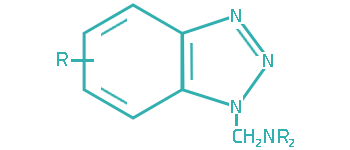
b. Chelators
The chelators, however, function in bulk of the lubricant by trapping metal ions to form an inactive or much less-active complex.
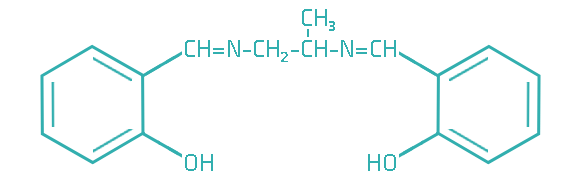
With either mechanism, metal deactivators can effectively slow the oxidation process catalyzed by those transitional metals, which in turn lends metal deactivators an antioxidant effect.
Destruction of alkyl radicals, alkyl peroxy radicals, and hydroperoxides
The destruction of alkyl radicals, alkyl peroxy radicals, and hydroperoxides can be achieved through the use of an appropriate antioxidant with …
a. Radical Scavengers – Primary Antioxidants
The radical scavengers function by donating hydrogen atoms to terminate alkoxy and alkyl peroxy radicals, thus interrupting the radical chain mechanism of the auto-oxidation process. The basis for a compound to become a successful antioxidant is that peroxy and alkoxyl radicals abstract hydrogen from the compound much more readily than they do from hydrocarbons. After hydrogen abstraction, the antioxidant becomes a stable radical, the alkyl radical becomes a hydrocarbon, and the alkyl peroxy radical becomes an alkyl hydroperoxide.
I. Hindered Phenolics
Phenols, especially the sterically hindered phenols, are class of antioxidants being extensively used in industrial and automotive lubricating oils and greases. Based on the chemical structure, phenols may be customarily categorized into simple phenols such as 2,6-di-tert-4- methylphenol (also known as BHT) and complex phenols that are typically in polymeric forms having molecular weights of 1000 or higher.
| Product Name | Structure | Melting Point | Solubility | Applications |
| 2,6-Di-tert-butyl-phenol | 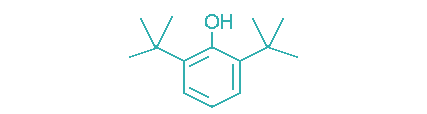 | 36–37 | >5 | Industrial oils, power transmission fluids, greases, fuels. |
| 2,6-Di-tert-butyl-4-methylphenol (BHT) | 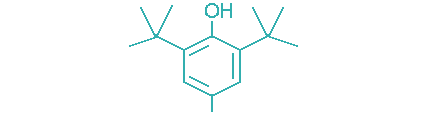 | 69 | >5 | Industrial oils, power transmission fluids, food grade lubricants, greases |
| (3,5-Di-tert-butyl-4-hydroxyhydrocinnamate)methane | 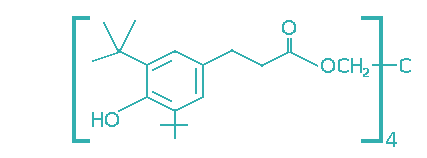 | 110- 125 | 0.1 – 0.5 | Industrial oils, food grade lubricants, greases. |
| Octadecyl 3-(3′,5′-di-tert-butyl-4′- hydroxyphenyl)propionate |  | 50 – 55 | >5 | Industrial oils, eco-friendly oils |
| 3,5-Di-tert-butyl-4-hydroxyhydrocinnamic acid, C7–C9 alkyl ester | 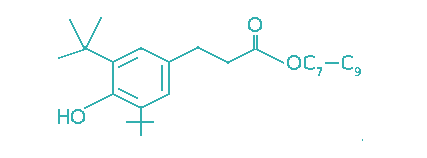 | 25 | >5 | Engine oils, power transmission fluids, industrial oils. |
| 3,5-Di-tert-butyl-4-hydrocinnamic acid, C13–C15 alkyl ester | 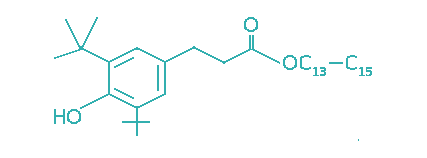 | 25 | >5 | Engine oils, power transmission fluids, industrial oils. |
| 4,4′-Methylene bis(2,6-di-tert-butylphenol) | 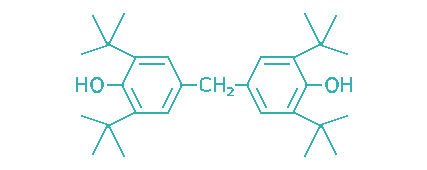 | 154 | NA | Engine oils, industrial oils, food grade lubricants, greases |
| 2,2′-Methylene bis(4-methyl-6-tert-butylphenol) | 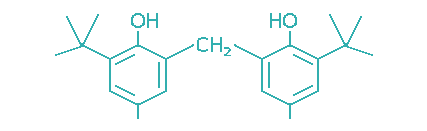 | 128 | 2 -5 | Engine oils, industrial oils, greases |
| 2-Propenoic acid, 3-[3,5-bis(1,1- dimethylethyl)-4-hydroxyphenyl]-1,6- hexanediyl ester | 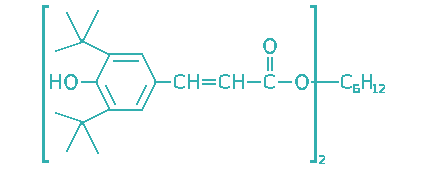 | 105 | <1 | Industrial oils, greases, food grade lubricants |
Example: 2,6-di-t-butyl-4- methylphenol, also known as BHT
The reaction of an alkyl radical with BHT versus oxygen shows that, the reaction rate constant (k2) of alkyl radical with oxygen to form alkyl peroxy radicals is much greater than that (k1) of alkyl radical with BHT.
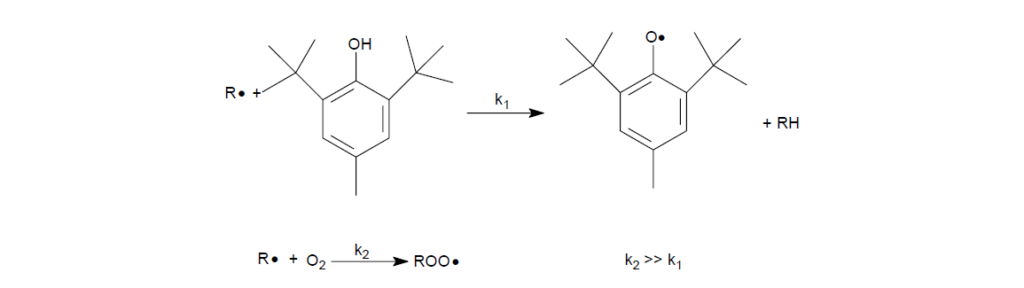
Hence with an ample supply of oxygen, the probability of BHT to react with alkyl radicals is low. As oxidation proceeds with more alkyl radicals being converted to alkyl peroxy radicals, BHT starts to react by donating a hydrogen atom to the peroxy radical. The peroxy radical is reduced to hydroperoxide, whereas the BHT is converted into a phenoxy radical that is stabilized through steric hindrance and resonance structures. The steric hindrance provided by the two butyl moieties on the ortho positions effectively prevent the phenoxy radical from attacking other hydrocarbons. The cyclohexadienone radical resonance structure can further combine with a second alkyl peroxy radical to form the alkyl peroxide, which is stable at temperatures <120°C.
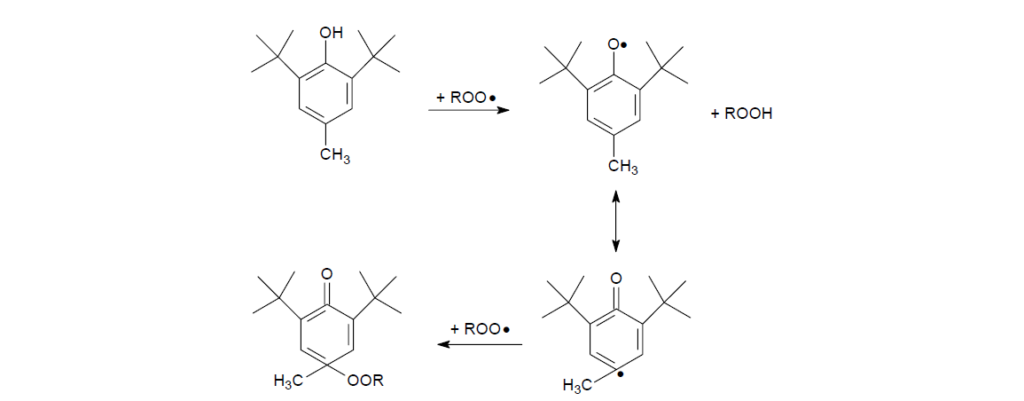
Without resonance transformation, an alternative reaction pathway for phenoxy radicals is to combine with each other into a chain termination step. The hydrogen atom is donated from one phenoxy radical to the other, thus regenerating one BHT molecule and one methylene cyclohexadienone.
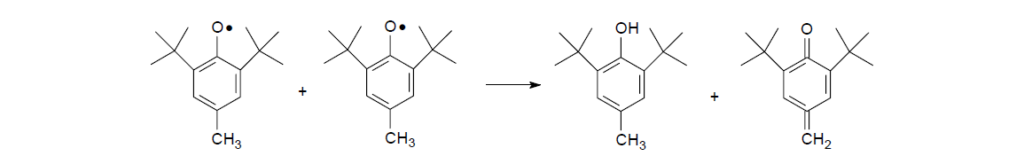
Under higher-temperature oxidation conditions, the cyclohexadienone alkyl peroxide previously formed is no longer stable. It will decompose to form an alkoxy radical, an alkyl radical, and a 2,6-di-t-butyl-1,4-benzoquinone. As can be expected, the generation of new radicals will deteriorate the overall effectiveness of the BHT under high-temperature oxidation conditions.
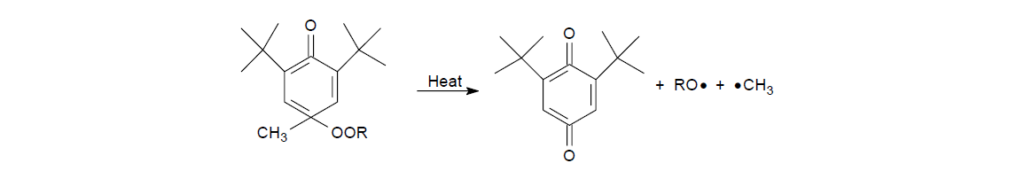
II. Aromatic Amines
Aromatic diphenylamine ADPAs are one of the most important classes of amine antioxidants being used today. Owing to their higher reactivity over the unsubstituted diphenylamine, ADPAs have been workhorse antioxidants for engine oils and various industrial lubricants.
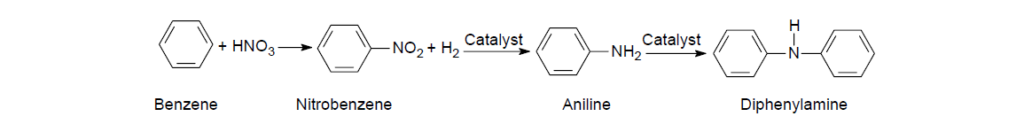
The typical synthesis routes of some commonly used ADPAs start with benzene, which is first converted into nitrobenzene, followed by a high-temperature reduction to aniline. Under very high-temperature (400–500°C) and high-pressure (50–150 psi) conditions, aniline can undergo a catalytic vapor-phase conversion to form diphenylamine.
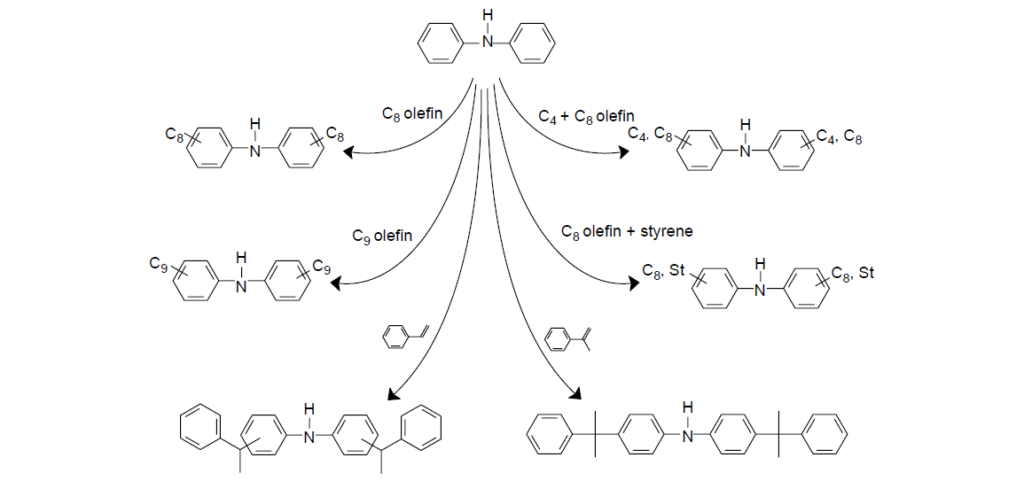
To make ADPAs, diphenylamine is reacted with an appropriate alkylating agent such as alcohol, alkyl halide, aliphatic carbonyl compound, or an olefin. The olefins are preferred for economic reason. The most commonly used are isobutylene (C4), diisobutylene (C8), nonenes (C9), styrene, and propylene tetramer (C12). Depending on the acidic catalyst, olefin, and other reaction conditions, for instance, the temperature, the degree of alkylation will vary from mono- to di-alkylation.
The reaction of the antioxidants begins with hydrogen atom abstraction by alkyl peroxy radical and alkoxy radical.

Owing to the rapid reaction of alkoxy radicals with oxygen, the resulting alkyl peroxy radical is present at higher concentration, and its reaction with ADPA predominates. The aminyl radical formed can undergo a number of possible reaction pathways depending on temperature, degree of oxidation (relative concentration of peroxy radicals versus alkyl radicals), and the chemical nature of the ADPA.
The low-temperature (<120°C) oxidation inhibition mechanism, starting with aminyl radical attacking a second alkyl peroxy radical to form a nitroxyl radical and alkoxy radical. The nitroxyl radical is stabilized through three possible resonance structures. Next, a third alkyl peroxy radical reacts with the nitroxyl radical to form a nitroxyl–peroxide complex, which can further eliminate an ether molecule, forming a nitroxyl cyclohexadienone. Following reactions involve a fourth alkyl peroxy radical being added to the nitroxyl cyclohexadienone to form nitroxyl cyclohexadienone peroxide, followed by a dissociation reaction to form 1,4-benzoquinone and an alkylated nitrosobenzene. Therefore, on an equal mole basis, in theory, ADPAs can quench twice as many alkyl peroxy radicals as a mono HP under low-temperature conditions.
At high temperatures (>120°C), the nitroxyl radical intermediate can undergo one of two possible reaction pathways by either reacting with a secondary or a tertiary alkyl radical to form an N-sec-alkoxy diphenylamine or an N-hydroxyl diphenylamine intermediate, respectively. In the former case, the resulting alkoxy amine intermediate can thermally rearrange to form a ketone and regenerate the starting ADPA. In the latter case, nitroxyl radical is regenerated upon reaction of the hydroxyl diphenylamine intermediate with an alkyl peroxy radical. Thus, at high temperatures, one molecule of ADPA can catalytically scavenge a large number of radicals before the nitroxyl radical is destroyed. It has been reported that such regeneration process can provide ADPAs with a stoichiometric efficiency of more than 12 radicals per molecule.
b. Peroxide Decomposers – Secondary Antioxidants
The peroxide decomposers are also called secondary antioxidants. They function by reducing alkyl hydroperoxides in the radical chain to nonradical, less-reactive alcohols.
I. Organosulfur Compounds
The antioxidant action starts with the reduction of an alkyl hydroperoxides to a less reactive alcohol, with the sulfide being oxidized to a sulfoxide intermediate.
A preferred mechanism for the subsequent reaction of sulfoxide intermediate is the intramolecular beta-hydrogen elimination, leading to the formation of a sulfenic acid (RSOH).
Sulfenic acid can (RSOH) further react with hydroperoxides to form sulfur-oxy acids.
At elevated temperatures, sulfininc acid (RSO2H) may decompose to form sulfur dioxide (SO2), which is a particularly powerful Lewis acid for hydroperoxide decomposition through the formations of active sulfur trioxide and sulfuric acid. Previous work has shown that one equivalent of SO2 was able to catalytically decompose up to 20,000 equivalents of cumene hydroperoxide.
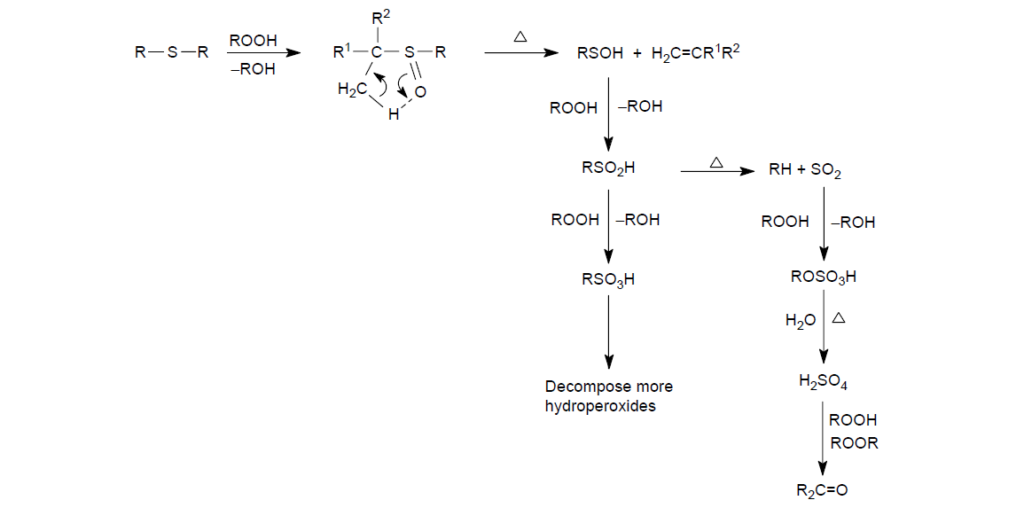
Further enhancing the antioxidancy of sulfur compounds is that, under certain conditions, the intermediate sulfur-oxy acids (RSOxH) can scavenge alkyl peroxy radicals, thus giving the sulfur compound primary antioxidant characteristics:
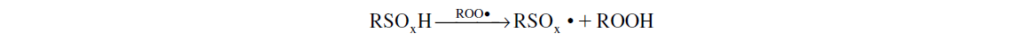
II. Organophosphorus Compounds
The good performance of phosphorus as an oxidation inhibitor in oils was identified early on in lubrication science. However, elemental phosphorus, like elemental sulfur, may have corrosive side effects to many nonferrous metals and alloys, so it is rarely incorporated in oils in this form, rather oil- soluble organic compounds of phosphorus are preferred. For optimum antioxidant performance, phosphites are customarily blended with aminic or HP antioxidants that can lead to synergistic effect.
| Phosphites | Applications |
| Trinonylphenyl phosphite, tributyl phosphite, tridecylphosphite, triphenylphosphite, trioctylphosphite, dilaurylphosphite | Compressor oils |
| Triaryl phosphites, trialkyl phosphites, alkyl aryl phosphites, acid dialkyl phosphites | Automotive and industrial lubricants |
| Triphenyl phosphite, diisodecyl pentaerythritol diphosphite, tri-isodecyl phosphite, dilauryl phosphite | Automotive and industrial lubricants |
| Steric hindered tributyl phosphite, bis(butylphenyl pentaerythritol) diphosphite | Hydraulic fluids, steam turbine oils, compressor oils, and heat-transfer oil |
| Triphenyl phosphite, trialkylsubstituted phenyl phosphite | Steam turbine oils, gas turbine oils |
| Trialkyl phosphites | Hydraulic fluids, Automatic transmission fluids |
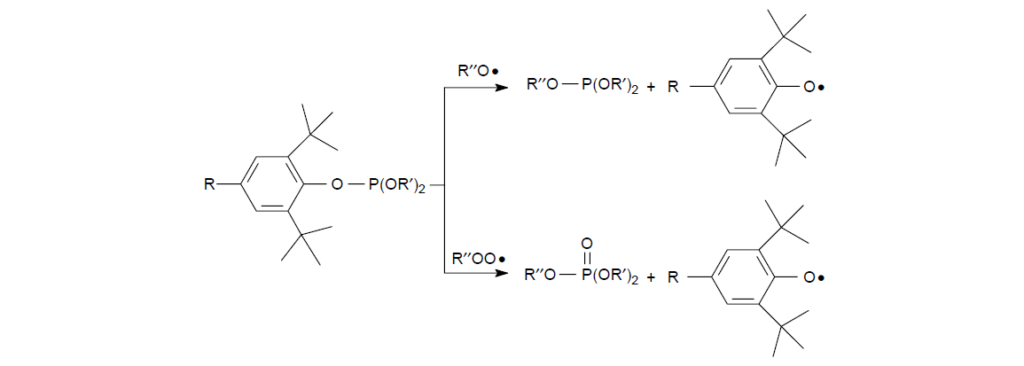
.Phosphites decompose hydroperoxides and peroxy radicals following the reaction mechanisms.
Phosphite is oxidized to corresponding phosphate, with the hydroperoxide and the peroxy radical being reduced to less-reactive alcohol and alkoxy radical, respectively.
Phosphites with certain substituted phenoxy groups may also behave as peroxy and alkoxy radical scavengers. The resulting phenoxy radicals can further eliminate peroxy radicals upon resonance transformation to cyclohyxadienone radical as discussed earlier. Owing to the steric hindrance provided by the alkyl groups on the ortho-positions, these phosphites tend to be more stable against hydrolysis and are preferred for use in moist lubrication environment.
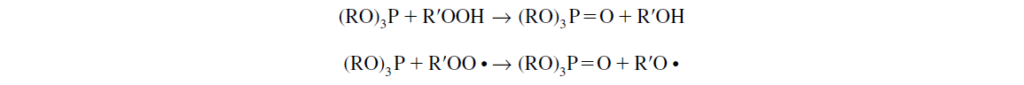
Antioxidant Synergism
Antioxidant synergism describes the effect or response of a combined use of two or more antioxidants being greater than that of any individual antioxidant. Synergistic antioxidant systems offer practical solutions to problems where using a single antioxidant is inadequate to provide satisfactory results, or where the treatment level has to be limited due to economic or environmental reasons. Three types of synergy have been proposed for lubricant antioxidants:
a. Homosynergism:
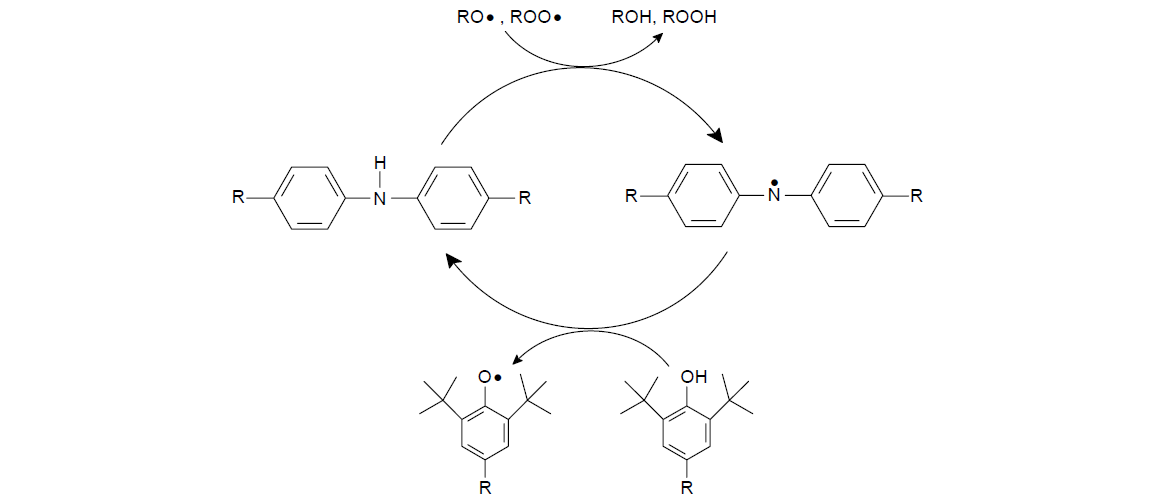
Homosynergism occurs when two antioxidants acting by the same mechanism interact, generally in a single-electron-transfer cascade. A classic example is an ADPA in combination with a HP antioxidant. ADPA is initially more reactive than HP in scavenging alkyl peroxy radicals.
For example, the amine is first converted to an aminyl radical, which is relatively less stable and will accept a hydrogen atom from the HP to regenerate the alkylated amine. In consequence, the HP is converted to a phenoxy radical. The driving force for this regeneration cycle to occur is the higher reactivity of the ADPA compared to the HP and the greater stability of the phenoxy radical relative to the aminyl radical. After the HP is consumed, the aromatic amine antioxidant begins to deplete. By regenerating the more reactive amine, the overall effectiveness of the system is enhanced, and the useful antioxidant lifetime is extended.
b. Heterosynergism
Heterosynergism occurs when antioxidants act by a different mechanism and hence complement each other. This type of synergy usually takes place when a primary antioxidant and a secondary antioxidant are present in one lubricant system. The primary antioxidant scavenges radicals, whereas the secondary antioxidant decomposes hydroperoxides by reducing them to more stable alcohols. Through these reactions, chain propagation and branching reactions are significantly slowed or inhibited. A representative example of a heterosynergism is an aminic antioxidant in combination with a zinc dialkyldithiophosphates ZDDP.
c. Autosynergism.
Autosynergism is a third type of synergistic response that results from two different antioxidant functions in the same molecule. Usually, antioxidants having functional groups that provide radical scavenging and hydroperoxide decomposing functions exhibit autosynergy. Examples of this type of antioxidants are sulfurized phenols and phenothiazines.

Facebook Comments